APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress
- PMID: 23754435
- PMCID: PMC3696815
- DOI: 10.1073/pnas.1301445110
APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress
Abstract
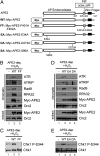
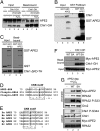
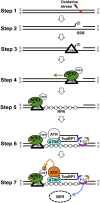
The base excision repair pathway is largely responsible for the repair of oxidative stress-induced DNA damage. However, it remains unclear how the DNA damage checkpoint is activated by oxidative stress at the molecular level. Here, we provide evidence showing that hydrogen peroxide (H2O2) triggers checkpoint kinase 1 (Chk1) phosphorylation in an ATR [ataxia-telangiectasia mutated (ATM) and Rad3-related]-dependent but ATM-independent manner in Xenopus egg extracts. A base excision repair protein, Apurinic/apyrimidinic (AP) endonuclease 2 (APE2, APN2, or APEX2), is required for the generation of replication protein A (RPA)-bound single-stranded DNA, the recruitment of a checkpoint protein complex [ATR, ATR-interacting protein (ATRIP), and Rad9] to damage sites, and H2O2-induced Chk1 phosphorylation. A conserved proliferating cell nuclear antigen interaction protein box of APE2 is important for the recruitment of APE2 to H2O2-damaged chromatin. APE2 3'-phosphodiesterase and 3'-5' exonuclease activity is essential for single-stranded DNA generation in the 3'-5' direction from single-stranded breaks, referred to as single-stranded break end resection. In addition, APE2 associates with Chk1, and a serine residue (S86) in the Chk1-binding motif of APE2 is essential for Chk1 phosphorylation, indicating a Claspin-like but distinct role for APE2 in ATR-Chk1 signaling. Our data indicate that APE2 plays a vital and previously unexpected role in ATR-Chk1 checkpoint signaling in response to oxidative stress. Thus, our findings shed light on a distinct mechanism of how an ATR-Chk1-dependent DNA damage checkpoint is mediated by APE2 in the oxidative stress response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
APE2 promotes DNA damage response pathway from a single-strand break.Nucleic Acids Res. 2018 Mar 16;46(5):2479-2494. doi: 10.1093/nar/gky020. Nucleic Acids Res. 2018. PMID: 29361157 Free PMC article.
-
Study of the DNA damage checkpoint using Xenopus egg extracts.J Vis Exp. 2012 Nov 5;(69):e4449. doi: 10.3791/4449. J Vis Exp. 2012. PMID: 23149695 Free PMC article.
-
Claspin and the activated form of ATR-ATRIP collaborate in the activation of Chk1.J Biol Chem. 2004 Nov 26;279(48):49599-608. doi: 10.1074/jbc.M408353200. Epub 2004 Sep 15. J Biol Chem. 2004. PMID: 15371427
-
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review.
-
Regulation of ATR-CHK1 signaling by ubiquitination of CLASPIN.Biochem Soc Trans. 2022 Oct 31;50(5):1471-1480. doi: 10.1042/BST20220729. Biochem Soc Trans. 2022. PMID: 36196914 Review.
Cited by
-
DNA damage: a sensible mediator of the differentiation decision in hematopoietic stem cells and in leukemia.Int J Mol Sci. 2015 Mar 17;16(3):6183-201. doi: 10.3390/ijms16036183. Int J Mol Sci. 2015. PMID: 25789504 Free PMC article. Review.
-
Oxidative stress, bone marrow failure, and genome instability in hematopoietic stem cells.Int J Mol Sci. 2015 Jan 22;16(2):2366-85. doi: 10.3390/ijms16022366. Int J Mol Sci. 2015. PMID: 25622253 Free PMC article. Review.
-
Claspin-Dependent and -Independent Chk1 Activation by a Panel of Biological Stresses.Biomolecules. 2023 Jan 7;13(1):125. doi: 10.3390/biom13010125. Biomolecules. 2023. PMID: 36671510 Free PMC article.
-
Cellular deficiency of Werner syndrome protein or RECQ1 promotes genotoxic potential of hydroquinone and benzo[a]pyrene exposure.Int J Toxicol. 2014 Sep-Oct;33(5):373-81. doi: 10.1177/1091581814547422. Epub 2014 Sep 15. Int J Toxicol. 2014. PMID: 25228686 Free PMC article.
-
Hyperosmotic stress induces 2-cell-like cells through ROS and ATR signaling.EMBO Rep. 2023 Sep 6;24(9):e56194. doi: 10.15252/embr.202256194. Epub 2023 Jul 11. EMBO Rep. 2023. PMID: 37432066 Free PMC article.
References
-
- Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38(1):96–109. - PubMed
-
- Friedberg EC. DNA damage and repair. Nature. 2003;421(6921):436–440. - PubMed
-
- Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

