Nox4-generated superoxide drives angiotensin II-induced neural stem cell proliferation
- PMID: 23751520
- PMCID: PMC3889712
- DOI: 10.1159/000350502
Nox4-generated superoxide drives angiotensin II-induced neural stem cell proliferation
Abstract
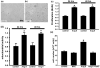
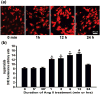
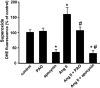
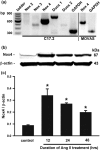
Reactive oxygen species (ROS) have been reported to affect neural stem cell self-renewal and therefore may be important for normal development and may influence neurodegenerative processes when ROS activity is elevated. To determine if increasing production of superoxide, via activation of NADPH oxidase (Nox), increases neural stem cell proliferation, 100 nM angiotensin II (Ang II) - a strong stimulator of Nox - was applied to cultures of a murine neural stem cell line, C17.2. Twelve hours following a single treatment with Ang II, there was a doubling of the number of neural stem cells. This increase in neural stem cell numbers was preceded by a gradual elevation of superoxide levels (detected by dihydroethidium fluorescence) from the steady state at 0, 5, and 30 min and gradually increasing from 1 h to the maximum at 12 h, and returning to baseline at 24 h. Ang II-dependent proliferation was blocked by the antioxidant N-acetyl-L-cysteine. Confocal microscopy revealed the presence of two sources of intracellular ROS in C17.2 cells: (i) mitochondrial and (ii) extramitochondrial; the latter indicative of the involvement of one or more specific isoforms of Nox. Of the Nox family, mRNA expression for one member, Nox4, is abundant in neural stem cell cultures, and Ang II treatment resulted in elevation of the relative levels of Nox4 protein. SiRNA targeting of Nox4 mRNA reduced both the constitutive and Ang II-induced Nox4 protein levels and attenuated Ang II-driven increases in superoxide levels and stem cell proliferation. Our findings are consistent with our hypothesis that Ang II-induced proliferation of neural stem cells occurs via Nox4-generated superoxide, suggesting that an Ang II/Nox4 axis is an important regulator of neural stem cell self-renewal and as such may fine-tune normal, stress- or disease-modifying neurogenesis.
Copyright © 2013 S. Karger AG, Basel.
Figures


Similar articles
-
Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: role of mitochondrial reactive oxygen species.J Biol Chem. 2013 Oct 4;288(40):28668-86. doi: 10.1074/jbc.M113.470971. Epub 2013 Aug 12. J Biol Chem. 2013. PMID: 23940049 Free PMC article.
-
Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons.Am J Physiol Heart Circ Physiol. 2013 Jul 1;305(1):H19-28. doi: 10.1152/ajpheart.00974.2012. Epub 2013 Apr 26. Am J Physiol Heart Circ Physiol. 2013. PMID: 23624625 Free PMC article.
-
Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells.PLoS One. 2012;7(7):e39739. doi: 10.1371/journal.pone.0039739. Epub 2012 Jul 9. PLoS One. 2012. PMID: 22808054 Free PMC article.
-
Angiotensin II, NADPH oxidase, and redox signaling in the vasculature.Antioxid Redox Signal. 2013 Oct 1;19(10):1110-20. doi: 10.1089/ars.2012.4641. Epub 2012 Jun 11. Antioxid Redox Signal. 2013. PMID: 22530599 Free PMC article. Review.
-
NOX Dependent ROS Generation and Cell Metabolism.Int J Mol Sci. 2023 Jan 20;24(3):2086. doi: 10.3390/ijms24032086. Int J Mol Sci. 2023. PMID: 36768405 Free PMC article. Review.
Cited by
-
Age-dependent alterations to paraventricular nucleus insulin-like growth factor 1 receptor as a possible link between sympathoexcitation and inflammation.J Neurochem. 2016 Dec;139(5):706-721. doi: 10.1111/jnc.13842. Epub 2016 Oct 19. J Neurochem. 2016. PMID: 27626839 Free PMC article.
-
Kinin B1 Receptor Mediates Renal Injury and Remodeling in Hypertension.Front Med (Lausanne). 2022 Jan 18;8:780834. doi: 10.3389/fmed.2021.780834. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35118089 Free PMC article.
-
Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity.J Exp Neurosci. 2016 Sep 4;10(Suppl 1):23-48. doi: 10.4137/JEN.S39887. eCollection 2016. J Exp Neurosci. 2016. PMID: 27625575 Free PMC article. Review.
-
NADPH Oxidases: Redox Regulators of Stem Cell Fate and Function.Antioxidants (Basel). 2021 Jun 17;10(6):973. doi: 10.3390/antiox10060973. Antioxidants (Basel). 2021. PMID: 34204425 Free PMC article. Review.
-
Crosstalk between Calcium and ROS in Pathophysiological Conditions.Oxid Med Cell Longev. 2019 Apr 24;2019:9324018. doi: 10.1155/2019/9324018. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31178978 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

