Neuronal-specific overexpression of a mutant valosin-containing protein associated with IBMPFD promotes aberrant ubiquitin and TDP-43 accumulation and cognitive dysfunction in transgenic mice
- PMID: 23747512
- PMCID: PMC3730785
- DOI: 10.1016/j.ajpath.2013.04.014
Neuronal-specific overexpression of a mutant valosin-containing protein associated with IBMPFD promotes aberrant ubiquitin and TDP-43 accumulation and cognitive dysfunction in transgenic mice
Abstract
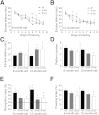
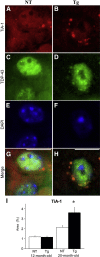
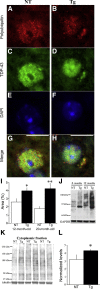
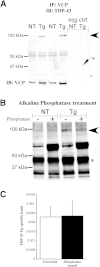
Mutations in valosin-containing protein (VCP) cause a rare, autosomal dominant disease called inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia (IBMPFD). One-third of patients with IBMPFD develop frontotemporal dementia, characterized by an extensive neurodegeneration in the frontal and temporal lobes. Neuropathologic hallmarks include nuclear and cytosolic inclusions positive to ubiquitin and transactive response DNA-binding protein 43 (TDP-43) in neurons and glial activation in affected regions. However, the pathogenic mechanisms by which mutant VCP triggers neurodegeneration remain unknown. Herein, we generated a mouse model selectively overexpressing a human mutant VCP in neurons to study pathogenic mechanisms of mutant VCP-mediated neurodegeneration and cognitive impairment. The overexpression of VCPA232E mutation in forebrain regions produced significant progressive impairments of cognitive function, including deficits in spatial memory, object recognition, and fear conditioning. Although overexpressed or endogenous VCP did not seem to focally aggregate inside neurons, TDP-43 and ubiquitin accumulated with age in transgenic mouse brains. TDP-43 was also found to co-localize with stress granules in the cytosolic compartment. Together with the appearance of high-molecular-weight TDP-43 in cytosolic fractions, these findings demonstrate the mislocalization and accumulation of abnormal TDP-43 in the cytosol of transgenic mice, which likely lead to an increase in cellular stress and cognitive impairment. Taken together, these results highlight an important pathologic link between VCP and cognition.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease.J Cell Biol. 2009 Dec 14;187(6):875-88. doi: 10.1083/jcb.200908115. J Cell Biol. 2009. PMID: 20008565 Free PMC article.
-
TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations.J Neuropathol Exp Neurol. 2007 Feb;66(2):152-7. doi: 10.1097/nen.0b013e31803020b9. J Neuropathol Exp Neurol. 2007. PMID: 17279000
-
Phenotypic variability in three families with valosin-containing protein mutation.Eur J Neurol. 2013 Feb;20(2):251-8. doi: 10.1111/j.1468-1331.2012.03831.x. Epub 2012 Aug 20. Eur J Neurol. 2013. PMID: 22900631 Free PMC article.
-
Valosin-containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto-temporal dementia.Neuromuscul Disord. 2009 May;19(5):308-15. doi: 10.1016/j.nmd.2009.01.009. Epub 2009 Apr 19. Neuromuscul Disord. 2009. PMID: 19380227 Free PMC article. Review.
-
The multiple faces of valosin-containing protein-associated diseases: inclusion body myopathy with Paget's disease of bone, frontotemporal dementia, and amyotrophic lateral sclerosis.J Mol Neurosci. 2011 Nov;45(3):522-31. doi: 10.1007/s12031-011-9627-y. Epub 2011 Sep 3. J Mol Neurosci. 2011. PMID: 21892620 Review.
Cited by
-
Neuronal VCP loss of function recapitulates FTLD-TDP pathology.Cell Rep. 2021 Jul 20;36(3):109399. doi: 10.1016/j.celrep.2021.109399. Cell Rep. 2021. PMID: 34289347 Free PMC article.
-
Ubiquitin-dependent proteolysis in yeast cells expressing neurotoxic proteins.Front Mol Neurosci. 2015 Mar 12;8:8. doi: 10.3389/fnmol.2015.00008. eCollection 2015. Front Mol Neurosci. 2015. PMID: 25814926 Free PMC article. Review.
-
Inherited and Sporadic Amyotrophic Lateral Sclerosis and Fronto-Temporal Lobar Degenerations arising from Pathological Condensates of Phase Separating Proteins.Hum Mol Genet. 2019 Nov 21;28(R2):R187-R196. doi: 10.1093/hmg/ddz162. Hum Mol Genet. 2019. PMID: 31595953 Free PMC article. Review.
-
P97/VCP ATPase inhibitors can rescue p97 mutation-linked motor neuron degeneration.Brain Commun. 2022 Jul 6;4(4):fcac176. doi: 10.1093/braincomms/fcac176. eCollection 2022. Brain Commun. 2022. PMID: 35865348 Free PMC article.
-
Cdc48/VCP and Endocytosis Regulate TDP-43 and FUS Toxicity and Turnover.Mol Cell Biol. 2020 Jan 30;40(4):e00256-19. doi: 10.1128/MCB.00256-19. Print 2020 Jan 30. Mol Cell Biol. 2020. PMID: 31767634 Free PMC article.
References
-
- Muller J.M., Meyer H.H., Ruhrberg C., Stamp G.W., Warren G., Shima D.T. The mouse p97 (CDC48) gene: genomic structure, definition of transcriptional regulatory sequences, gene expression, and characterization of a pseudogene. J Biol Chem. 1999;274:10154–10162. - PubMed
-
- Hirabayashi M., Inoue K., Tanaka K., Nakadate K., Ohsawa Y., Kamei Y., Popiel A.H., Sinohara A., Iwamatsu A., Kimura Y., Uchiyama Y., Hori S., Kakizuka A. VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 2001;8:977–984. - PubMed
-
- Wang Q., Song C., Li C.C. Hexamerization of p97-VCP is promoted by ATP binding to the D1 domain and required for ATPase and biological activities. Biochem Biophys Res Commun. 2003;300:253–260. - PubMed
-
- Wang Q., Song C., Li C.C. Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol. 2004;146:44–57. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

