Vascular targeting to the SST2 receptor improves the therapeutic response to near-IR two-photon activated PDT for deep-tissue cancer treatment
- PMID: 23747302
- PMCID: PMC3764460
- DOI: 10.1016/j.bbagen.2013.05.043
Vascular targeting to the SST2 receptor improves the therapeutic response to near-IR two-photon activated PDT for deep-tissue cancer treatment
Abstract
Background: Broader clinical acceptance of photodynamic therapy is currently hindered by (a) poor depth efficacy, and (b) predisposition towards establishment of an angiogenic environment during the treatment. Improved depth efficacy is being sought by exploiting the NIR tissue transparency window and by photo-activation using two-photon absorption (2PA). Here, we use two-photon activation of PDT sensitizers, untargeted and targeted to SST2 receptors or EGF receptors, to achieve deep tissue treatment.
Methods: Human tumor lines, positive or negative for SST2r expression were used, as well as murine 3LL cells and bovine aortic endothelial cells. Expression of SST2 receptors on cancer cells and tumor vasculature was evaluated in vitro and frozen xenograft sections. PDT effects on tumor blood flow were followed using in vivo scanning after intravenous injection of FITC conjugated dextran 150K. Dependence of the PDT efficacy on the laser pulse duration was evaluated. Effectiveness of targeting to vascular SST2 receptors was compared to that of EGF receptors, or no targeting.
Results: Tumor vasculature stained for SST2 receptors even in tumors from SST2 receptor negative cell lines, and SST2r targeted PDT led to tumor vascular shutdown. Stretching the pulse from ~120fs to ~3ps led to loss of the PDT efficacy especially at greater depth. PDT targeted to SST2 receptors was much more effective than untargeted PDT or PDT targeted to EGF receptors.
General significance: The use of octreotate to target SST2 receptors expressed on tumor vessels is an excellent approach to PDT with few recurrences and some long term cures.
Keywords: 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride; ATCC; American type culture collection; BAE; CMF; DMEM; Dulbecco's minimal essential medium; EDC; EDTA; EGF; Ethylenediaminetetraacetic acid; FITC; GE11 peptide; Laser pulse; NIR; PBS; PDT; Photodynamic therapy; SCID; SST2r; Somatostatin receptor 2; UCNP; VEGF; Vascular shutdown; YHWYGYTPQNVI; bovine aortic endothelial; calcium and magnesium free saline; epidermal growth factor; fluorescein isothiocyanate; near infrared; phosphate buffered saline; photodynamic therapy; severe combined immunodeficient; somatostatin type 2 receptor; upconversion nanoparticle; vascular endothelial growth factor.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report the following potential conflicts of interest: A.K. Rebane and J.R. Starkey have 8.49% and 4.72% ownership interests respectively in SensoPath Technologies Inc.
Figures

 treated directly =
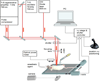
treated directly =  Panel A = FaDu xenograft subcutaneous tumors induced as described in materials and methods, treated using our normal femtosecond laser pulse protocol for PDT. Panel B = FaDu xenograft subcutaneous tumors induced as described in materials and methods, treated using 10 fold pulse elongation of the laser beam. Panel C = 3LL subcutaneous tumors induced as described in materials and methods, treated using 10 fold pulse elongation of the laser beam. Note that the last day possible for tumor measurement for the group treated through the mouse was day +8 after laser treatment. This was due to aggressive growth of the tumor in this group.
Panel A = FaDu xenograft subcutaneous tumors induced as described in materials and methods, treated using our normal femtosecond laser pulse protocol for PDT. Panel B = FaDu xenograft subcutaneous tumors induced as described in materials and methods, treated using 10 fold pulse elongation of the laser beam. Panel C = 3LL subcutaneous tumors induced as described in materials and methods, treated using 10 fold pulse elongation of the laser beam. Note that the last day possible for tumor measurement for the group treated through the mouse was day +8 after laser treatment. This was due to aggressive growth of the tumor in this group.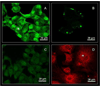

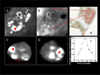

 , tumors received PDT using sensitizer targeted to the EGF receptor =
, tumors received PDT using sensitizer targeted to the EGF receptor =  , tumors received PDT using sensitizer targeted to the SST2 receptor =
, tumors received PDT using sensitizer targeted to the SST2 receptor =  tumors received PDT using a 50:50 mixture of sensitizer targeted to the EGF receptor and sensitizer targeted to the SST2 receptor =
tumors received PDT using a 50:50 mixture of sensitizer targeted to the EGF receptor and sensitizer targeted to the SST2 receptor =  . Panel B) tumors received no treatment (control group for groups treated with targeted sensitizers in panel A) =
. Panel B) tumors received no treatment (control group for groups treated with targeted sensitizers in panel A) =  , tumors received no treatment (control group for the group treated using untargeted sensitizer in panel A) =
, tumors received no treatment (control group for the group treated using untargeted sensitizer in panel A) =  .
.Similar articles
-
Somatostatin analogues for receptor targeted photodynamic therapy.PLoS One. 2014 Aug 11;9(8):e104448. doi: 10.1371/journal.pone.0104448. eCollection 2014. PLoS One. 2014. PMID: 25111655 Free PMC article.
-
New two-photon activated photodynamic therapy sensitizers induce xenograft tumor regressions after near-IR laser treatment through the body of the host mouse.Clin Cancer Res. 2008 Oct 15;14(20):6564-73. doi: 10.1158/1078-0432.CCR-07-4162. Clin Cancer Res. 2008. PMID: 18927297
-
Targeted Delivery of Functionalized Upconversion Nanoparticles for Externally Triggered Photothermal/Photodynamic Therapies of Brain Glioblastoma.Theranostics. 2018 Feb 4;8(5):1435-1448. doi: 10.7150/thno.22482. eCollection 2018. Theranostics. 2018. PMID: 29507632 Free PMC article.
-
Vascular and cellular targeting for photodynamic therapy.Crit Rev Eukaryot Gene Expr. 2006;16(4):279-305. doi: 10.1615/critreveukargeneexpr.v16.i4.10. Crit Rev Eukaryot Gene Expr. 2006. PMID: 17206921 Review.
-
Carbon nanotubes for delivery of small molecule drugs.Adv Drug Deliv Rev. 2013 Dec;65(15):1964-2015. doi: 10.1016/j.addr.2013.08.005. Epub 2013 Aug 14. Adv Drug Deliv Rev. 2013. PMID: 23954402 Review.
Cited by
-
EDTA-Modified 17β-Estradiol-Laden Upconversion Nanocomposite for Bone-Targeted Hormone Replacement Therapy for Osteoporosis.Theranostics. 2020 Feb 10;10(7):3281-3292. doi: 10.7150/thno.37599. eCollection 2020. Theranostics. 2020. PMID: 32194868 Free PMC article.
-
Photodynamic viral inactivation: Recent advances and potential applications.Appl Phys Rev. 2021 Jun;8(2):021315. doi: 10.1063/5.0044713. Appl Phys Rev. 2021. PMID: 34084253 Free PMC article. Review.
-
The somatostatin analog 188Re-P2045 inhibits the growth of AR42J pancreatic tumor xenografts.J Nucl Med. 2014 Dec;55(12):2020-5. doi: 10.2967/jnumed.114.140780. Epub 2014 Oct 30. J Nucl Med. 2014. PMID: 25359879 Free PMC article.
-
Encapsulation of Hydrophobic Porphyrins into Biocompatible Nanoparticles: An Easy Way to Benefit of Their Two-Photon Phototherapeutic Effect without Hydrophilic Functionalization.Cancers (Basel). 2022 May 10;14(10):2358. doi: 10.3390/cancers14102358. Cancers (Basel). 2022. PMID: 35625963 Free PMC article.
-
Anti-tumor immunity of BAM-SiPc-mediated vascular photodynamic therapy in a BALB/c mouse model.Cell Mol Immunol. 2017 Feb;14(2):223-234. doi: 10.1038/cmi.2015.84. Epub 2015 Sep 21. Cell Mol Immunol. 2017. PMID: 26388236 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

