Sexually selected traits: a fundamental framework for studies on behavioral epigenetics
- PMID: 23744965
- PMCID: PMC3679548
- DOI: 10.1093/ilar.53.3-4.253
Sexually selected traits: a fundamental framework for studies on behavioral epigenetics
Abstract
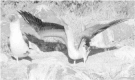
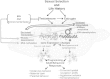
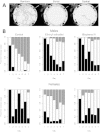
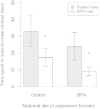
Emerging evidence suggests that epigenetic-based mechanisms contribute to various aspects of sex differences in brain and behavior. The major obstacle in establishing and fully understanding this linkage is identifying the traits that are most susceptible to epigenetic modification. We have proposed that sexual selection provides a conceptual framework for identifying such traits. These are traits involved in intrasexual competition for mates and intersexual choice of mating partners and generally entail a combination of male-male competition and female choice. These behaviors are programmed during early embryonic and postnatal development, particularly during the transition from the juvenile to adult periods, by exposure of the brain to steroid hormones, including estradiol and testosterone. We evaluate the evidence that endocrine-disrupting compounds, including bisphenol A, can interfere with the vital epigenetic and gene expression pathways and with the elaboration of sexually selected traits with epigenetic mechanisms presumably governing the expression of these traits. Finally, we review the evidence to suggest that these steroid hormones can induce a variety of epigenetic changes in the brain, including the extent of DNA methylation, histone protein alterations, and even alterations of noncoding RNA, and that many of the changes differ between males and females. Although much previous attention has focused on primary sex differences in reproductive behaviors, such as male mounting and female lordosis, we outline why secondary sex differences related to competition and mate choice might also trace their origins back to steroid-induced epigenetic programming in disparate regions of the brain.
Keywords: DNA methylation; histone proteins; neurodevelopment; sex dimorphism; steroid hormones.
Figures




Similar articles
-
Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility.Prog Brain Res. 2010;186:77-95. doi: 10.1016/B978-0-444-53630-3.00006-3. Prog Brain Res. 2010. PMID: 21094887 Free PMC article. Review.
-
Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A.Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11715-20. doi: 10.1073/pnas.1107958108. Epub 2011 Jun 27. Proc Natl Acad Sci U S A. 2011. PMID: 21709224 Free PMC article.
-
Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us.Horm Behav. 1996 Dec;30(4):627-61. doi: 10.1006/hbeh.1996.0066. Horm Behav. 1996. PMID: 9047287 Review.
-
Past, present and future of epigenetics in brain sexual differentiation.J Neuroendocrinol. 2018 Feb;30(2). doi: 10.1111/jne.12492. J Neuroendocrinol. 2018. PMID: 28585265 Review.
-
Epigenetic impacts of endocrine disruptors in the brain.Front Neuroendocrinol. 2017 Jan;44:1-26. doi: 10.1016/j.yfrne.2016.09.002. Epub 2016 Sep 20. Front Neuroendocrinol. 2017. PMID: 27663243 Free PMC article. Review.
Cited by
-
Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism.Endocr Rev. 2014 Dec;35(6):961-91. doi: 10.1210/er.2013-1122. Epub 2014 Sep 11. Endocr Rev. 2014. PMID: 25211453 Free PMC article. Review.
-
Should Pregnant Women Consume Probiotics to Combat Endocrine-Disrupting Chemical-Induced Health Risks to Their Unborn Offspring?Biomedicines. 2024 Jul 23;12(8):1628. doi: 10.3390/biomedicines12081628. Biomedicines. 2024. PMID: 39200093 Free PMC article. Review.
-
Neuroendocrine disruption of organizational and activational hormone programming in poikilothermic vertebrates.J Toxicol Environ Health B Crit Rev. 2017;20(5):276-304. doi: 10.1080/10937404.2017.1370083. J Toxicol Environ Health B Crit Rev. 2017. PMID: 28895797 Free PMC article. Review.
-
Data integration, analysis, and interpretation of eight academic CLARITY-BPA studies.Reprod Toxicol. 2020 Dec;98:29-60. doi: 10.1016/j.reprotox.2020.05.014. Epub 2020 Jul 16. Reprod Toxicol. 2020. PMID: 32682780 Free PMC article.
-
Developmental exposure of California mice to endocrine disrupting chemicals and potential effects on the microbiome-gut-brain axis at adulthood.Sci Rep. 2020 Jul 2;10(1):10902. doi: 10.1038/s41598-020-67709-9. Sci Rep. 2020. PMID: 32616744 Free PMC article.
References
-
- Adkins-Regan E. 2005. Hormones and Animal Social Behavior. Princeton NJ: Princeton University Press
-
- Amaral PP, Dinger ME, Mercer TR, Mattick JS. 2008. The eukaryotic genome as an RNA machine. Science 319:1787–1789. - PubMed
-
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. 1999. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23:185–188. - PubMed
-
- Amundsen T. 2000. Why are female birds ornamented? Trends Ecol Evol 15:149–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

