B7-1/B7-2 blockade overrides the activation of protective CD8 T cells stimulated in the absence of Foxp3+ regulatory T cells
- PMID: 23744647
- PMCID: PMC3714566
- DOI: 10.1189/jlb.0313118
B7-1/B7-2 blockade overrides the activation of protective CD8 T cells stimulated in the absence of Foxp3+ regulatory T cells
Abstract
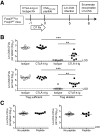
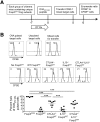
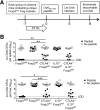
Although T cell activation has been classically described to require distinct, positive stimulation signals that include B7-1 (CD80) and B7-2 (CD86) costimulation, overriding suppression signals that avert immune-mediated host injury are equally important. How these opposing stimulation and suppression signals work together remains incompletely defined. Our recent studies demonstrate that CD8 Teff activation in response to cognate peptide stimulation is actively suppressed by the Foxp3(+) subset of CD4 cells, called Tregs. Here, we show that the elimination of Treg suppression does not bypass the requirement for positive B7-1/B7-2 costimulation. The expansion, IFN-γ cytokine production, cytolytic, and protective features of antigen-specific CD8 T cells stimulated with purified cognate peptide in Treg-ablated mice were each neutralized effectively by CTLA-4-Ig that blocks B7-1/B7-2. In turn, given the efficiency whereby CTLA-4-Ig overrides the effects of Treg ablation, the role of Foxp3(+) cell-intrinsic CTLA-4 in mitigating CD8 Teff activation was also investigated. With the use of mixed chimera mice that contain CTLA-4-deficient Tregs exclusively after the ablation of WT Foxp3(+) cells, a critical role for Treg CTLA-4 in suppressing the expansion, cytokine production, cytotoxicity, and protective features of peptide-stimulated CD8 T cells is revealed. Thus, the activation of protective CD8 T cells requires positive B7-1/B7-2 costimulation even when suppression by Tregs and in particular, Treg-intrinsic CTLA-4 is circumvented.
Keywords: Treg; bacterial; costimulation; cytotoxic T cells.
Figures




Similar articles
-
Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells.Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2023739118. doi: 10.1073/pnas.2023739118. Proc Natl Acad Sci U S A. 2021. PMID: 34301886 Free PMC article.
-
Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T-cells.PLoS One. 2013 Dec 23;8(12):e83139. doi: 10.1371/journal.pone.0083139. eCollection 2013. PLoS One. 2013. PMID: 24376655 Free PMC article.
-
The role of B7 costimulation in CD4/CD8 T cell homeostasis.J Immunol. 2000 Apr 1;164(7):3543-53. doi: 10.4049/jimmunol.164.7.3543. J Immunol. 2000. PMID: 10725709
-
Targeting T cell costimulation in autoimmune disease.Expert Opin Ther Targets. 2002 Jun;6(3):275-89. doi: 10.1517/14728222.6.3.275. Expert Opin Ther Targets. 2002. PMID: 12223069 Review.
-
Treg and CTLA-4: two intertwining pathways to immune tolerance.J Autoimmun. 2013 Sep;45(100):49-57. doi: 10.1016/j.jaut.2013.06.006. Epub 2013 Jul 10. J Autoimmun. 2013. PMID: 23849743 Free PMC article. Review.
Cited by
-
IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion.J Cereb Blood Flow Metab. 2013 Dec;33(12):1955-66. doi: 10.1038/jcbfm.2013.155. Epub 2013 Sep 11. J Cereb Blood Flow Metab. 2013. PMID: 24022622 Free PMC article.
-
Perinatal Listeria monocytogenes susceptibility despite preconceptual priming and maintenance of pathogen-specific CD8(+) T cells during pregnancy.Cell Mol Immunol. 2014 Nov;11(6):595-605. doi: 10.1038/cmi.2014.84. Epub 2014 Sep 22. Cell Mol Immunol. 2014. PMID: 25242275 Free PMC article.
-
Immunogenic, but not steady-state, antigen presentation permits regulatory T-cells to control CD8+ T-cell effector differentiation by IL-2 modulation.PLoS One. 2014 Jan 13;9(1):e85455. doi: 10.1371/journal.pone.0085455. eCollection 2014. PLoS One. 2014. PMID: 24454872 Free PMC article.
-
Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2.Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):10672-7. doi: 10.1073/pnas.1402336111. Epub 2014 Jul 7. Proc Natl Acad Sci U S A. 2014. PMID: 25002484 Free PMC article.
-
Regulatory T cells and the immune pathogenesis of prenatal infection.Reproduction. 2013 Oct 21;146(6):R191-203. doi: 10.1530/REP-13-0262. Print 2013 Dec. Reproduction. 2013. PMID: 23929902 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

