Extracellular domains of CD8α and CD8ß subunits are sufficient for HLA class I restricted helper functions of TCR-engineered CD4(+) T cells
- PMID: 23738014
- PMCID: PMC3667802
- DOI: 10.1371/journal.pone.0065212
Extracellular domains of CD8α and CD8ß subunits are sufficient for HLA class I restricted helper functions of TCR-engineered CD4(+) T cells
Abstract
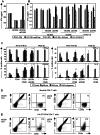
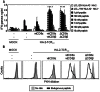
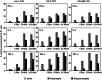
By gene transfer of HLA-class I restricted T-cell receptors (TCRs) (HLA-I-TCR) into CD8(+) as well as CD4(+) T-cells, both effector T-cells as well as helper T-cells can be generated. Since most HLA-I-TCRs function best in the presence of the CD8 co-receptor, the CD8αß molecule has to be co-transferred into the CD4(+) T-cells to engineer optimal helper T-cells. In this study, we set out to determine the minimal part of CD8αβ needed for optimal co-receptor function in HLA-I-TCR transduced CD4(+) T-cells. For this purpose, we transduced human peripheral blood derived CD4(+) T-cells with several HLA-class I restricted TCRs either with or without co-transfer of different CD8 subunits. We demonstrate that the co-transduced CD8αβ co-receptor in HLA-I-TCR transduced CD4(+) T-cells behaves as an adhesion molecule, since for optimal antigen-specific HLA class I restricted CD4(+) T-cell reactivity the extracellular domains of the CD8α and ß subunits are sufficient.
Conflict of interest statement
Figures
Similar articles
-
HLA class II restricted T-cell receptor gene transfer generates CD4+ T cells with helper activity as well as cytotoxic capacity.Gene Ther. 2005 Dec;12(23):1686-95. doi: 10.1038/sj.gt.3302586. Gene Ther. 2005. PMID: 16034453
-
Redirecting human CD4+ T lymphocytes to the MHC class I-restricted melanoma antigen MAGE-A1 by TCR alphabeta gene transfer requires CD8alpha.Gene Ther. 2005 Jan;12(2):140-6. doi: 10.1038/sj.gt.3302388. Gene Ther. 2005. PMID: 15496961
-
A rare population of tumor antigen-specific CD4+CD8+ double-positive αβ T lymphocytes uniquely provide CD8-independent TCR genes for engineering therapeutic T cells.J Immunother Cancer. 2019 Jan 9;7(1):7. doi: 10.1186/s40425-018-0467-y. J Immunother Cancer. 2019. PMID: 30626427 Free PMC article.
-
CD4 Helper and CD8 Cytotoxic T Cell Differentiation.Annu Rev Immunol. 2018 Apr 26;36:579-601. doi: 10.1146/annurev-immunol-042617-053411. Annu Rev Immunol. 2018. PMID: 29677476 Review.
-
Interplay between the TCR/CD3 complex and CD4 or CD8 in the activation of cytotoxic T lymphocytes.Immunol Rev. 1989 Jun;109:119-41. doi: 10.1111/j.1600-065x.1989.tb00022.x. Immunol Rev. 1989. PMID: 2527803 Review.
Cited by
-
Antileukemia multifunctionality of CD4(+) T cells genetically engineered by HLA class I-restricted and WT1-specific T-cell receptor gene transfer.Leukemia. 2015 Dec;29(12):2393-401. doi: 10.1038/leu.2015.155. Epub 2015 Jun 24. Leukemia. 2015. PMID: 26104661
-
A T-cell reporter platform for high-throughput and reliable investigation of TCR function and biology.Clin Transl Immunology. 2020 Nov 23;9(11):e1216. doi: 10.1002/cti2.1216. eCollection 2020. Clin Transl Immunology. 2020. PMID: 33251011 Free PMC article.
-
Engineering Strategies to Enhance TCR-Based Adoptive T Cell Therapy.Cells. 2020 Jun 18;9(6):1485. doi: 10.3390/cells9061485. Cells. 2020. PMID: 32570906 Free PMC article. Review.
-
Cognate CD4 T-cell licensing of dendritic cells heralds anti-cytomegalovirus CD8 T-cell immunity after human allogeneic umbilical cord blood transplantation.J Virol. 2015 Jan 15;89(2):1058-69. doi: 10.1128/JVI.01850-14. Epub 2014 Nov 5. J Virol. 2015. PMID: 25378489 Free PMC article.
-
Single-cell transcriptomics identifies multiple pathways underlying antitumor function of TCR- and CD8αβ-engineered human CD4+ T cells.Sci Adv. 2020 Jul 3;6(27):eaaz7809. doi: 10.1126/sciadv.aaz7809. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32923584 Free PMC article.
References
-
- Collins RH Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, et al. (1997) Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 15: 433–444. - PubMed
-
- Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, et al. (1990) Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 76: 2462–2465. - PubMed
-
- Porter DL, Collins RH Jr, Hardy C, Kernan NA, Drobyski WR, et al. (2000) Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood 95: 1214–1221. - PubMed
-
- Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, et al. (1999) Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol 163: 507–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

