Interferon- γ triggers hepatic stellate cell-mediated immune regulation through MEK/ERK signaling pathway
- PMID: 23737812
- PMCID: PMC3662191
- DOI: 10.1155/2013/389807
Interferon- γ triggers hepatic stellate cell-mediated immune regulation through MEK/ERK signaling pathway
Abstract
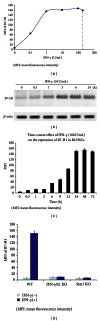
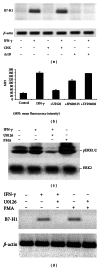
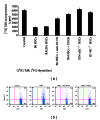
Hepatic stellate cells (HSCs) interact with immune cells to actively participate in regulating immune response in the liver which is mediated by the effector molecules, including B7-H1. We demonstrated here that expression of B7-H1 on HSCs was markedly enhanced by interferon-(IFN-) γ stimulation. IFN- γ stimulated HSCs inhibited T-cell proliferation via induction of T-cell apoptosis (22.1% ± 1.6%). This immunosuppressive effect was inhibited by preincubation with an anti-B7-H1 antibody, or inhibitor of the MEK/ERK pathway inhibited IFN- γ mediated expression of B7-H1. Thus, regulation of B7-H1 expression on HSCs by IFN- γ represents an important mechanism that regulates immune responses in the liver favoring tolerogenicity rather than immunogenicity. Involvement of MEK/ERK pathway provides a novel target for therapeutic approaches.
Figures
Similar articles
-
Human hepatic stellate cells inhibit T-cell response through B7-H1 pathway.Transplantation. 2013 Jul 15;96(1):17-24. doi: 10.1097/TP.0b013e318294caae. Transplantation. 2013. PMID: 23756770 Free PMC article.
-
Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway.Blood. 2007 Jul 1;110(1):296-304. doi: 10.1182/blood-2006-10-051482. Epub 2007 Mar 15. Blood. 2007. PMID: 17363736
-
Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-gamma signaling.Hepatology. 2009 Dec;50(6):1981-91. doi: 10.1002/hep.23202. Hepatology. 2009. PMID: 19821484 Free PMC article.
-
Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice.Hepatology. 2004 Dec;40(6):1312-21. doi: 10.1002/hep.20488. Hepatology. 2004. PMID: 15565659
-
1-Methyl-L-tryptophan promotes the apoptosis of hepatic stellate cells arrested by interferon-γ by increasing the expression of IFN-γRβ, IRF-1 and FAS.Int J Mol Med. 2017 Aug;40(2):576-582. doi: 10.3892/ijmm.2017.3043. Epub 2017 Jun 26. Int J Mol Med. 2017. PMID: 28656203
Cited by
-
Interferon-α Up-Regulates the Expression of PD-L1 Molecules on Immune Cells Through STAT3 and p38 Signaling.Front Immunol. 2018 Sep 27;9:2129. doi: 10.3389/fimmu.2018.02129. eCollection 2018. Front Immunol. 2018. PMID: 30356906 Free PMC article.
-
Soluble FGL2, a novel effector molecule of activated hepatic stellate cells, regulates T-cell function in cirrhotic patients with hepatocellular carcinoma.Hepatol Int. 2014 Sep 25;8(4):567-75. doi: 10.1007/s12072-014-9568-y. eCollection 2014 Oct. Hepatol Int. 2014. PMID: 25298849 Free PMC article.
-
Therapeutic Targeting of the Tumour Microenvironment in Metastatic Colorectal Cancer.Int J Mol Sci. 2021 Feb 19;22(4):2067. doi: 10.3390/ijms22042067. Int J Mol Sci. 2021. PMID: 33669775 Free PMC article. Review.
-
Hepatic Stellate Cells Inhibit T Cells through Active TGF-β1 from a Cell Surface-Bound Latent TGF-β1/GARP Complex.J Immunol. 2015 Sep 15;195(6):2648-56. doi: 10.4049/jimmunol.1500139. Epub 2015 Aug 5. J Immunol. 2015. PMID: 26246140 Free PMC article.
-
Betulinic acid attenuates liver fibrosis by inducing autophagy via the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway.J Nat Med. 2019 Jan;73(1):179-189. doi: 10.1007/s11418-018-1262-2. Epub 2018 Oct 30. J Nat Med. 2019. PMID: 30377904
References
-
- Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223(5205):472–476. - PubMed
-
- Farges O, Morris PJ, Dallman MJ. Spontaneous acceptance of liver allografts in the rat: analysis of the immune response. Transplantation. 1994;57(2):171–177. - PubMed
-
- Ilan Y, Sauter B, Chowdhury NR, et al. Oral tolerization to adenoviral proteins permits repeated adenovirus- mediated gene therapy in rats with pre-existing immunity to adenoviruses. Hepatology. 1998;27(5):1368–1376. - PubMed
-
- Yu S, Nakafusa Y, Flye MW, et al. Portal vein administration of donor cells promotes peripheral allospecific hyporesponsiveness and graft tolerance. Surgery. 1994;116(2):229–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

