Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle
- PMID: 23733748
- PMCID: PMC3752537
- DOI: 10.1096/fj.13-230375
Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle
Abstract
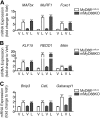
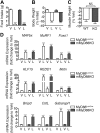
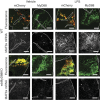
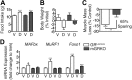
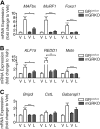
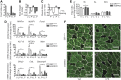
Cachexia is a wasting condition defined by skeletal muscle atrophy in the setting of systemic inflammation. To explore the site at which inflammatory mediators act to produce atrophy in vivo, we utilized mice with a conditional deletion of the inflammatory adaptor protein myeloid differentiation factor 88 (MyD88). Although whole-body MyD88-knockout (wbMyD88KO) mice resist skeletal muscle atrophy in response to LPS, muscle-specific deletion of MyD88 is not protective. Furthermore, selective reexpression of MyD88 in the muscle of wbMyD88KO mice via electroporation fails to restore atrophy gene induction by LPS. To evaluate the role of glucocorticoids as the inflammation-induced mediator of atrophy in vivo, we generated mice with targeted deletion of the glucocorticoid receptor in muscle (mGRKO mice). Muscle-specific deletion of the glucocorticoid receptor affords a 71% protection against LPS-induced atrophy compared to control animals. Furthermore, mGRKO mice exhibit 77% less skeletal muscle atrophy than control animals in response to tumor growth. These data demonstrate that glucocorticoids are a major determinant of inflammation-induced atrophy in vivo and play a critical role in the pathogenesis of endotoxemic and cancer cachexia.
Keywords: MyD88; adenocarcinoma; atrophy; corticosterone; inflammation; lipopolysaccharide; sickness behavior.
Figures


Similar articles
-
Muscle atrophy in response to cytotoxic chemotherapy is dependent on intact glucocorticoid signaling in skeletal muscle.PLoS One. 2014 Sep 25;9(9):e106489. doi: 10.1371/journal.pone.0106489. eCollection 2014. PLoS One. 2014. PMID: 25254959 Free PMC article.
-
The Toll-Like Receptor/MyD88/XBP1 Signaling Axis Mediates Skeletal Muscle Wasting during Cancer Cachexia.Mol Cell Biol. 2019 Jul 16;39(15):e00184-19. doi: 10.1128/MCB.00184-19. Print 2019 Aug 1. Mol Cell Biol. 2019. PMID: 31138662 Free PMC article.
-
Cancer-Induced Muscle Wasting Requires p38β MAPK Activation of p300.Cancer Res. 2021 Feb 15;81(4):885-897. doi: 10.1158/0008-5472.CAN-19-3219. Epub 2020 Dec 22. Cancer Res. 2021. PMID: 33355181 Free PMC article.
-
Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions.Biochim Biophys Acta Rev Cancer. 2020 Apr;1873(2):188359. doi: 10.1016/j.bbcan.2020.188359. Epub 2020 Mar 25. Biochim Biophys Acta Rev Cancer. 2020. PMID: 32222610 Free PMC article. Review.
-
Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia.Int J Biochem Cell Biol. 2013 Oct;45(10):2322-32. doi: 10.1016/j.biocel.2013.05.035. Epub 2013 Jun 13. Int J Biochem Cell Biol. 2013. PMID: 23769949 Free PMC article. Review.
Cited by
-
TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice.Sci Rep. 2020 Jan 20;10(1):694. doi: 10.1038/s41598-020-57714-3. Sci Rep. 2020. PMID: 31959927 Free PMC article.
-
Cancer cachexia: understanding the molecular basis.Nat Rev Cancer. 2014 Nov;14(11):754-62. doi: 10.1038/nrc3829. Epub 2014 Oct 9. Nat Rev Cancer. 2014. PMID: 25291291 Review.
-
Role of Glucocorticoid Signaling and HDAC4 Activation in Diaphragm and Gastrocnemius Proteolytic Activity in Septic Rats.Int J Mol Sci. 2022 Mar 26;23(7):3641. doi: 10.3390/ijms23073641. Int J Mol Sci. 2022. PMID: 35408999 Free PMC article.
-
MyD88 signalling is critical in the development of pancreatic cancer cachexia.J Cachexia Sarcopenia Muscle. 2019 Apr;10(2):378-390. doi: 10.1002/jcsm.12377. Epub 2019 Jan 21. J Cachexia Sarcopenia Muscle. 2019. PMID: 30666818 Free PMC article.
-
Understanding cachexia in the context of metastatic progression.Nat Rev Cancer. 2020 May;20(5):274-284. doi: 10.1038/s41568-020-0251-4. Epub 2020 Mar 31. Nat Rev Cancer. 2020. PMID: 32235902 Review.
References
-
- Evans W. K., Makuch R., Clamon G. H., Feld R., Weiner R. S., Moran E., Blum R., Shepherd F. A., Jeejeebhoy K. N., DeWys W. D. (1985) Limited impact of total parenteral nutrition on nutritional status during treatment for small cell lung cancer. Cancer Res. 45, 3347–3353 - PubMed
-
- DeWys W. D. (1986) Weight loss and nutritional abnormalities in cancer patients: incidence, severity and significance. Clinics Oncol. 5, 251–261
-
- Tan B. H., Birdsell L. A., Martin L., Baracos V. E., Fearon K. C. (2009) Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin. Cancer Res. 15, 6973–6979 - PubMed
-
- Tisdale M. J. (2002) Cachexia in cancer patients. Nat. Rev. Cancer 2, 862–871 - PubMed
-
- Baracos V. E., DeVivo C., Hoyle D. H., Goldberg A. L. (1995) Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am. J. Physiol. Endocrinol. Metab. Physiol. 268, E996–E1006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

