Response of striosomal opioid signaling to dopamine depletion in 6-hydroxydopamine-lesioned rat model of Parkinson's disease: a potential compensatory role
- PMID: 23730270
- PMCID: PMC3656348
- DOI: 10.3389/fncel.2013.00074
Response of striosomal opioid signaling to dopamine depletion in 6-hydroxydopamine-lesioned rat model of Parkinson's disease: a potential compensatory role
Abstract
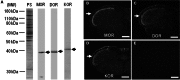
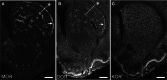
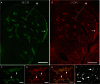
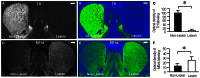
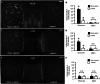
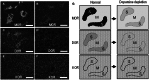
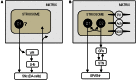
The opioid peptide receptors consist of three major subclasses, namely, μ, δ, and κ (MOR, DOR, and KOR, respectively). They are involved in the regulation of striatal dopamine functions, and increased opioid transmissions are thought to play a compensatory role in altered functions of the basal ganglia in Parkinson's disease (PD). In this study, we used an immunohistochemistry with tyramide signal amplification (TSA) protocols to determine the distributional patterns of opioid receptors in the striosome-matrix systems of the rat striatum. As a most striking feature of striatal opioid anatomy, MORs are highly enriched in the striosomes and subcallosal streak. We also found that DORs are localized in a mosaic pattern in the dorsal striatum (caudate-putamen), with heightened labeling for DOR in the striosomes relative to the matrix compartment. In the 6-hydroxydopamine-lesioned rat model of PD, lesions of the nigrostriatal pathways caused a significant reduction of striatal labeling for both the MOR and DOR in the striosomes, but not in the matrix compartment. Our results suggest that the activities of the striosome and matrix compartments are differentially regulated by the opioid signals involving the MORs and DORs, and that the striosomes may be more responsive to opioid peptides (e.g., enkephalin) than the matrix compartment. Based on a model in which the striosome compartment regulates the striatal activity, we propose a potent compensatory role of striosomal opioid signaling under the conditions of the striatal dopamine depletion that occurs in PD.
Keywords: Parkinson's disease; dopamine; opioid receptors; striatum; striosomes.
Figures

Similar articles
-
Impact of 6-hydroxydopamine lesions and cocaine exposure on mu-opioid receptor expression and regulation of cholinergic transmission in the limbic-prefrontal territory of the rat dorsal striatum.Eur J Neurosci. 2007 Mar;25(5):1546-56. doi: 10.1111/j.1460-9568.2007.05375.x. Eur J Neurosci. 2007. PMID: 17425581
-
Compartment-specific modulation of GABAergic synaptic transmission by mu-opioid receptor in the mouse striatum with green fluorescent protein-expressing dopamine islands.J Neurosci. 2007 Sep 5;27(36):9721-8. doi: 10.1523/JNEUROSCI.2993-07.2007. J Neurosci. 2007. PMID: 17804632 Free PMC article.
-
Preferential cytoplasmic localization of delta-opioid receptors in rat striatal patches: comparison with plasmalemmal mu-opioid receptors.J Neurosci. 2001 May 1;21(9):3242-50. doi: 10.1523/JNEUROSCI.21-09-03242.2001. J Neurosci. 2001. PMID: 11312309 Free PMC article.
-
Roles of micro-opioid receptors in GABAergic synaptic transmission in the striosome and matrix compartments of the striatum.Mol Neurobiol. 2008 Apr-Jun;37(2-3):104-15. doi: 10.1007/s12035-008-8023-2. Epub 2008 May 13. Mol Neurobiol. 2008. PMID: 18473190 Review.
-
Striosomes and Matrisomes: Scaffolds for Dynamic Coupling of Volition and Action.Annu Rev Neurosci. 2023 Jul 10;46:359-380. doi: 10.1146/annurev-neuro-121522-025740. Epub 2023 Apr 17. Annu Rev Neurosci. 2023. PMID: 37068787 Review.
Cited by
-
Spinal Central Effects of Peripherally Applied Botulinum Neurotoxin A in Comparison between Its Subtypes A1 and A2.Front Neurol. 2014 Jun 23;5:98. doi: 10.3389/fneur.2014.00098. eCollection 2014. Front Neurol. 2014. PMID: 25002857 Free PMC article.
-
Development of a highly sensitive immunohistochemical method to detect neurochemical molecules in formalin-fixed and paraffin-embedded tissues from autopsied human brains.Front Neuroanat. 2015 Mar 3;9:22. doi: 10.3389/fnana.2015.00022. eCollection 2015. Front Neuroanat. 2015. PMID: 25784860 Free PMC article.
-
Compartmental function and modulation of the striatum.J Neurosci Res. 2019 Dec;97(12):1503-1514. doi: 10.1002/jnr.24522. Epub 2019 Sep 5. J Neurosci Res. 2019. PMID: 31489687 Free PMC article. Review.
-
A novel tyrosine kinase inhibitor AMN107 (nilotinib) normalizes striatal motor behaviors in a mouse model of Parkinson's disease.Front Cell Neurosci. 2014 Feb 20;8:50. doi: 10.3389/fncel.2014.00050. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24600352 Free PMC article.
-
The delta-opioid receptor and Parkinson's disease.CNS Neurosci Ther. 2018 Dec;24(12):1089-1099. doi: 10.1111/cns.13045. Epub 2018 Aug 3. CNS Neurosci Ther. 2018. PMID: 30076686 Free PMC article. Review.
References
-
- Alexander G. E., Crutcher M. D. (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

