Control of both myeloid cell infiltration and angiogenesis by CCR1 promotes liver cancer metastasis development in mice
- PMID: 23730212
- PMCID: PMC3664996
- DOI: 10.1593/neo.121866
Control of both myeloid cell infiltration and angiogenesis by CCR1 promotes liver cancer metastasis development in mice
Abstract
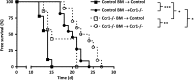
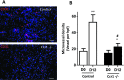
Expression of the CC chemokine receptor 1 (CCR1) by tumor cells has been associated with protumoral activity; however, its role in nontumoral cells during tumor development remains elusive. Here, we investigated the role of CCR1 deletion on stromal and hematopoietic cells in a liver metastasis tumor model. Metastasis development was strongly impaired in CCR1-deficient mice compared to control mice and was associated with reduced liver monocyte infiltration. To decipher the role of myeloid cells, sublethally irradiated mice were reconstituted with CCR1-deficient bone marrow (BM) and showed better survival rates than the control reconstituted mice. These results point toward the involvement of CCR1 myeloid cell infiltration in the promotion of tumor burden. In addition, survival rates were extended in CCR1-deficient mice receiving either control or CCR1-deficient BM, indicating that host CCR1 expression on nonhematopoietic cells also supports tumor growth. Finally, we found defective tumor-induced neoangiogenesis (in vitro and in vivo) in CCR1-deficient mice. Overall, our results indicate that CCR1 expression by both hematopoietic and nonhematopoietic cells favors tumor aggressiveness. We propose CCR1 as a potential therapeutical target for liver metastasis therapy.
Figures
Similar articles
-
Synergistic antitumor activity by dual blockade of CCR1 and CXCR2 expressed on myeloid cells within the tumor microenvironment.Br J Cancer. 2024 Jul;131(1):63-76. doi: 10.1038/s41416-024-02710-x. Epub 2024 May 15. Br J Cancer. 2024. PMID: 38750114 Free PMC article.
-
Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis.Gastroenterology. 2013 Nov;145(5):1064-1075.e11. doi: 10.1053/j.gastro.2013.07.033. Epub 2013 Jul 25. Gastroenterology. 2013. PMID: 23891973
-
Disruption of CCR1-mediated myeloid cell accumulation suppresses colorectal cancer progression in mice.Cancer Lett. 2020 Sep 1;487:53-62. doi: 10.1016/j.canlet.2020.05.028. Epub 2020 May 27. Cancer Lett. 2020. PMID: 32473241
-
CCR1-mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis.Clin Exp Metastasis. 2014 Dec;31(8):977-89. doi: 10.1007/s10585-014-9684-z. Epub 2014 Oct 18. Clin Exp Metastasis. 2014. PMID: 25326065 Free PMC article.
-
Molecular mechanisms of liver metastasis.Int J Clin Oncol. 2011 Oct;16(5):464-72. doi: 10.1007/s10147-011-0307-2. Epub 2011 Aug 17. Int J Clin Oncol. 2011. PMID: 21847533 Review.
Cited by
-
Effect of Chemokine (C-C Motif) Ligand 7 (CCL7) and Its Receptor (CCR2) Expression on Colorectal Cancer Behaviors.Int J Mol Sci. 2019 Feb 5;20(3):686. doi: 10.3390/ijms20030686. Int J Mol Sci. 2019. PMID: 30764543 Free PMC article.
-
Chemokines and their receptors play important roles in the development of hepatocellular carcinoma.World J Hepatol. 2015 Jun 8;7(10):1390-402. doi: 10.4254/wjh.v7.i10.1390. World J Hepatol. 2015. PMID: 26052384 Free PMC article. Review.
-
A high-throughput in vivo screening method in the mouse for identifying regulators of metastatic colonization.Nat Protoc. 2017 Dec;12(12):2465-2477. doi: 10.1038/nprot.2017.118. Epub 2017 Nov 2. Nat Protoc. 2017. PMID: 29095442
-
The Role of Chemokines in Promoting Colorectal Cancer Invasion/Metastasis.Int J Mol Sci. 2016 Apr 28;17(5):643. doi: 10.3390/ijms17050643. Int J Mol Sci. 2016. PMID: 27136535 Free PMC article. Review.
-
Cancer subclonal genetic architecture as a key to personalized medicine.Neoplasia. 2013 Dec;15(12):1410-20. doi: 10.1593/neo.131972. Neoplasia. 2013. PMID: 24403863 Free PMC article.
References
-
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. - PubMed
-
- Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T, Schettini G. Stromal cell-derived factor 1α stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 2003;63:1969–1974. - PubMed
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. - PubMed
-
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
