Improved clearance during treatment of HPV-positive head and neck cancer through mTOR inhibition
- PMID: 23730210
- PMCID: PMC3664994
- DOI: 10.1593/neo.13432
Improved clearance during treatment of HPV-positive head and neck cancer through mTOR inhibition
Abstract
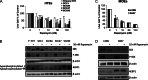
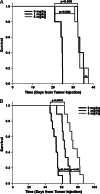
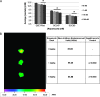
Human papillomavirus (HPV)-related head and neck squamous cell carcinoma (HNSCC) incidence is increasing at a near epidemic rate. We investigated whether the mammalian (or mechanistic) target of rapamycin (mTOR) inhibitor, rapamycin, can be used as a concurrent agent to standard-of-care cisplatin/radiation therapy (CRT) to attenuate tumor lactate production, thus enhancing CRT-induced immune-mediated clearance of this antigenic tumor type. A C57Bl/6-derived mouse oropharyngeal epithelial cell line retrovirally transduced with HPV type 16 E6/E7 and human squamous cell carcinoma cell lines were evaluated for their response to rapamycin in vitro with proliferation assays, Western blots, and lactate assays. Clonogenic assays and a preclinical mouse model were used to assess rapamycin as a concurrent agent to CRT. The potential of rapamycin to enhance immune response through lactate attenuation was assessed using quantitative tumor lactate bioluminescence and assessment of cell-mediated immunity using E6/E7-vaccinated mouse splenocytes. Rapamycin alone inhibited mTOR signaling of all cancer cell lines tested in vitro and in vivo. Furthermore, rapamycin administered alone significantly prolonged survival in vivo but did not result in any long-term cures. Given concurrently, CRT/rapamycin significantly enhanced direct cell killing in clonogenic assays and prolonged survival in immunocompromised mice. However, in immunocompetent mice, concurrent CRT/rapamycin increased long-term cures by 21%. Preliminary findings suggest that improved survival involves increased cell killing and enhanced immune-mediated clearance in part due to decreased lactate production. The results may provide rationale for the clinical evaluation of mTOR inhibitors concurrent with standard-of-care CRT for treatment of HPV-positive HNSCC.
Figures



Similar articles
-
mTOR inhibition as an adjuvant therapy in a metastatic model of HPV+ HNSCC.Oncotarget. 2016 Apr 26;7(17):24228-41. doi: 10.18632/oncotarget.8286. Oncotarget. 2016. PMID: 27015118 Free PMC article.
-
Impact of human papilloma virus infection on the response of head and neck cancers to anti-epidermal growth factor receptor antibody therapy.Cell Death Dis. 2014 Feb 27;5(2):e1091. doi: 10.1038/cddis.2014.62. Cell Death Dis. 2014. PMID: 24577089 Free PMC article.
-
Combination of melatonin and rapamycin for head and neck cancer therapy: Suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation.J Pineal Res. 2018 Apr;64(3). doi: 10.1111/jpi.12461. Epub 2018 Jan 9. J Pineal Res. 2018. PMID: 29247557
-
Mammalian target of rapamycin and head and neck squamous cell carcinoma.Head Neck Oncol. 2011 Apr 24;3:22. doi: 10.1186/1758-3284-3-22. Head Neck Oncol. 2011. PMID: 21513566 Free PMC article. Review.
-
The interplay between HPV and host immunity in head and neck squamous cell carcinoma.Int J Cancer. 2014 Jun 15;134(12):2755-63. doi: 10.1002/ijc.28411. Epub 2013 Aug 29. Int J Cancer. 2014. PMID: 23913554 Review.
Cited by
-
Increased Growth of a Newly Established Mouse Epithelial Cell Line Transformed with HPV-16 E7 in Diabetic Mice.PLoS One. 2016 Oct 17;11(10):e0164490. doi: 10.1371/journal.pone.0164490. eCollection 2016. PLoS One. 2016. PMID: 27749912 Free PMC article.
-
Induction of dormancy in hypoxic human papillomavirus-positive cancer cells.Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):E990-E998. doi: 10.1073/pnas.1615758114. Epub 2017 Jan 23. Proc Natl Acad Sci U S A. 2017. PMID: 28115701 Free PMC article.
-
Dichloroacetate and Quercetin Prevent Cell Proliferation, Induce Cell Death and Slow Tumor Growth in a Mouse Model of HPV-Positive Head and Neck Cancer.Cancers (Basel). 2024 Apr 17;16(8):1525. doi: 10.3390/cancers16081525. Cancers (Basel). 2024. PMID: 38672607 Free PMC article.
-
mTOR, metabolism, and the immune response in HPV-positive head and neck squamous cell cancer.World J Otorhinolaryngol Head Neck Surg. 2016 Jul 20;2(2):76-83. doi: 10.1016/j.wjorl.2016.05.010. eCollection 2016 Jun. World J Otorhinolaryngol Head Neck Surg. 2016. PMID: 29204551 Free PMC article. Review.
-
Propranolol Promotes Glucose Dependence and Synergizes with Dichloroacetate for Anti-Cancer Activity in HNSCC.Cancers (Basel). 2018 Nov 30;10(12):476. doi: 10.3390/cancers10120476. Cancers (Basel). 2018. PMID: 30513596 Free PMC article.
References
-
- American Cancer Society, author. Cancer Facts & Figures 2011. Atlanta, GA: American Cancer Society; 2011.
-
- Dufour X, Beby-Defaux A, Agius G, Lacau St Guily J. HPV and head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:26–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
