Detailed topology mapping reveals substantial exposure of the "cytoplasmic" C-terminal tail (CTT) sequences in HIV-1 Env proteins at the cell surface
- PMID: 23724133
- PMCID: PMC3664582
- DOI: 10.1371/journal.pone.0065220
Detailed topology mapping reveals substantial exposure of the "cytoplasmic" C-terminal tail (CTT) sequences in HIV-1 Env proteins at the cell surface
Abstract
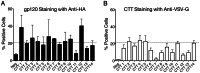
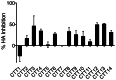
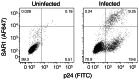
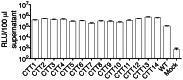
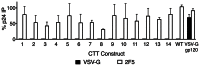
Substantial controversy surrounds the membrane topology of the HIV-1 gp41 C-terminal tail (CTT). While few studies have been designed to directly address the topology of the CTT, results from envelope (Env) protein trafficking studies suggest that the CTT sequence is cytoplasmically localized, as interactions with intracellular binding partners are required for proper Env targeting. However, previous studies from our lab demonstrate the exposure of a short CTT sequence, the Kennedy epitope, at the plasma membrane of intact Env-expressing cells, the exposure of which is not observed on viral particles. To address the topology of the entire CTT sequence, we serially replaced CTT sequences with a VSV-G epitope tag sequence and examined reactivity of cell- and virion-surface Env to an anti-VSV-G monoclonal antibody. Our results demonstrate that the majority of the CTT sequence is accessible to antibody binding on the surface of Env expressing cells, and that the CTT-exposed Env constitutes 20-50% of the cell-surface Env. Cell surface CTT exposure was also apparent in virus-infected cells. Passive transfer of Env through cell culture media to Env negative (non-transfected) cells was not responsible for the apparent cell surface CTT exposure. In contrast to the cell surface results, CTT-exposed Env was not detected on infectious pseudoviral particles containing VSV-G-substituted Env. Finally, a monoclonal antibody directed to the Kennedy epitope neutralized virus in a temperature-dependent manner in a post-attachment neutralization assay. Collectively, these results suggest that the membrane topology of the HIV gp41 CTT is more complex than the widely accepted intracytoplasmic model.
Conflict of interest statement
Figures



Similar articles
-
Topology of the C-terminal tail of HIV-1 gp41: differential exposure of the Kennedy epitope on cell and viral membranes.PLoS One. 2010 Dec 7;5(12):e15261. doi: 10.1371/journal.pone.0015261. PLoS One. 2010. PMID: 21151874 Free PMC article.
-
C-terminal tail of human immunodeficiency virus gp41: functionally rich and structurally enigmatic.J Gen Virol. 2013 Jan;94(Pt 1):1-19. doi: 10.1099/vir.0.046508-0. Epub 2012 Oct 17. J Gen Virol. 2013. PMID: 23079381 Free PMC article. Review.
-
A plasma membrane localization signal in the HIV-1 envelope cytoplasmic domain prevents localization at sites of vesicular stomatitis virus budding and incorporation into VSV virions.Virology. 1998 Nov 25;251(2):244-52. doi: 10.1006/viro.1998.9429. Virology. 1998. PMID: 9837788
-
Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M.J Virol. 2000 Aug;74(15):7096-107. doi: 10.1128/jvi.74.15.7096-7107.2000. J Virol. 2000. PMID: 10888650 Free PMC article.
-
The frantic play of the concealed HIV envelope cytoplasmic tail.Retrovirology. 2013 May 24;10:54. doi: 10.1186/1742-4690-10-54. Retrovirology. 2013. PMID: 23705972 Free PMC article. Review.
Cited by
-
The Interplay between HIV-1 Gag Binding to the Plasma Membrane and Env Incorporation.Viruses. 2020 May 16;12(5):548. doi: 10.3390/v12050548. Viruses. 2020. PMID: 32429351 Free PMC article. Review.
-
Recombinant expression, purification, and biophysical characterization of the transmembrane and membrane proximal domains of HIV-1 gp41.Protein Sci. 2014 Nov;23(11):1607-18. doi: 10.1002/pro.2540. Epub 2014 Sep 3. Protein Sci. 2014. PMID: 25155369 Free PMC article.
-
Cell surface ectodomain integrity of a subset of functional HIV-1 envelopes is dependent on a conserved hydrophilic domain containing region in their C-terminal tail.Retrovirology. 2018 Jul 20;15(1):50. doi: 10.1186/s12977-018-0431-4. Retrovirology. 2018. PMID: 30029604 Free PMC article.
-
Dense Array of Spikes on HIV-1 Virion Particles.J Virol. 2017 Jun 26;91(14):e00415-17. doi: 10.1128/JVI.00415-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28446665 Free PMC article.
-
Physics of HIV.J Phys D Appl Phys. 2018 May 10;51(18):183001. doi: 10.1088/1361-6463/aab731. Epub 2018 Apr 12. J Phys D Appl Phys. 2018. PMID: 34552276 Free PMC article.
References
-
- Luciw PA (2002) Fields' Virology; Fields BN, Knipe DM, Howley PM, et al... Philadelphia: Lippincott-Raven.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

