Similar dose-dependence of motor neuron cell death caused by wild type human TDP-43 and mutants with ALS-associated amino acid substitutions
- PMID: 23721326
- PMCID: PMC3684520
- DOI: 10.1186/1423-0127-20-33
Similar dose-dependence of motor neuron cell death caused by wild type human TDP-43 and mutants with ALS-associated amino acid substitutions
Abstract
Background: TDP-43, a multi-functional DNA/ RNA-binding protein encoded by the TARDBP gene, has emerged as a major patho-signature factor of the ubiquitinated intracellular inclusions (UBIs) in the diseased cells of a range of neurodegenerative diseases. Mutations in at least 9 different genes including TARDBP have been identified in ALS with TDP-43 (+)-UBIs. Thus far, the pathogenic role(s) of the more than 30 ALS-associated mutations in the TARDBP gene has not been well defined.
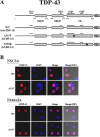
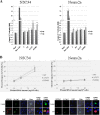
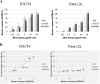
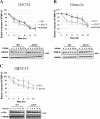
Results: By transient DNA transfection studies, we show that exogenously expressed human TDP-43 (hTDP-43), either wild type (WT) or 2 different ALS mutant (MT) forms, could cause significantly higher apoptotic death rate of a mouse spinal motor neuron-like cell line (NSC34) than other types of cells, e.g. mouse neuronal Neuro2a and human fibroblast HEK293T cells. Furthermore, at the same plasmid DNA dose(s) used for transfection, the percentages of NSC34 cell death caused by the 2 exogenously expressed hTDP-43 mutants are all higher than that caused by the WT hTDP-43. Significantly, the above observations are correlated with higher steady-state levels of the mutant hTDP-43 proteins as well as their stabilities than the WT.
Conclusions: Based on these data and previous transgenic TDP-43 studies in animals or cell cultures, we suggest that one major common consequence of the different ALS-associated TDP-43 mutations is the stabilization of the hTDP-43 polypeptide. The resulting elevation of the steady state level of hTDP-43 in combination with the relatively low tolerance of the spinal motor neurons to the increased amount of hTDP-43 lead to the neurodegeneration and pathogenesis of ALS, and of diseases with TDP-43 proteinopathies in general.
Figures
Similar articles
-
Human TDP-43 and FUS selectively affect motor neuron maturation and survival in a murine cell model of ALS by non-cell-autonomous mechanisms.Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(7-8):431-41. doi: 10.3109/21678421.2015.1055275. Epub 2015 Sep 7. Amyotroph Lateral Scler Frontotemporal Degener. 2015. PMID: 26174443
-
Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo.Hum Mol Genet. 2010 Feb 15;19(4):671-83. doi: 10.1093/hmg/ddp534. Epub 2009 Dec 3. Hum Mol Genet. 2010. PMID: 19959528
-
Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice.J Clin Invest. 2011 Feb;121(2):726-38. doi: 10.1172/JCI44867. Epub 2011 Jan 4. J Clin Invest. 2011. PMID: 21206091 Free PMC article.
-
Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis.Hum Mol Genet. 2009 Oct 15;18(R2):R156-62. doi: 10.1093/hmg/ddp303. Hum Mol Genet. 2009. PMID: 19808791 Free PMC article. Review.
-
[Recent progress in ALS research: ALS and TDP-43].Rinsho Shinkeigaku. 2008 Sep;48(9):625-33. doi: 10.5692/clinicalneurol.48.625. Rinsho Shinkeigaku. 2008. PMID: 19048944 Review. Japanese.
Cited by
-
TDP-43 Proteinopathy and ALS: Insights into Disease Mechanisms and Therapeutic Targets.Neurotherapeutics. 2015 Apr;12(2):352-63. doi: 10.1007/s13311-015-0338-x. Neurotherapeutics. 2015. PMID: 25652699 Free PMC article. Review.
-
Structural analysis of disease-related TDP-43 D169G mutation: linking enhanced stability and caspase cleavage efficiency to protein accumulation.Sci Rep. 2016 Feb 17;6:21581. doi: 10.1038/srep21581. Sci Rep. 2016. PMID: 26883171 Free PMC article.
-
A robust TDP-43 knock-in mouse model of ALS.Acta Neuropathol Commun. 2020 Jan 21;8(1):3. doi: 10.1186/s40478-020-0881-5. Acta Neuropathol Commun. 2020. PMID: 31964415 Free PMC article.
-
Quantification of the Relative Contributions of Loss-of-function and Gain-of-function Mechanisms in TAR DNA-binding Protein 43 (TDP-43) Proteinopathies.J Biol Chem. 2016 Sep 9;291(37):19437-48. doi: 10.1074/jbc.M116.737726. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445339 Free PMC article.
-
TARDBP Mutations in Facial-Onset Sensory and Motor Neuronopathy.Neurol Genet. 2024 Jun 4;10(3):e200160. doi: 10.1212/NXG.0000000000200160. eCollection 2024 Jun. Neurol Genet. 2024. PMID: 38841627 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

