Calcium signaling in the liver
- PMID: 23720295
- PMCID: PMC3986042
- DOI: 10.1002/cphy.c120013
Calcium signaling in the liver
Abstract
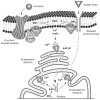
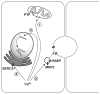
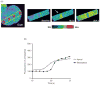
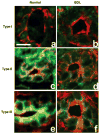
Intracellular free Ca(2+) ([Ca(2+)]i) is a highly versatile second messenger that regulates a wide range of functions in every type of cell and tissue. To achieve this versatility, the Ca(2+) signaling system operates in a variety of ways to regulate cellular processes that function over a wide dynamic range. This is particularly well exemplified for Ca(2+) signals in the liver, which modulate diverse and specialized functions such as bile secretion, glucose metabolism, cell proliferation, and apoptosis. These Ca(2+) signals are organized to control distinct cellular processes through tight spatial and temporal coordination of [Ca(2+)]i signals, both within and between cells. This article will review the machinery responsible for the formation of Ca(2+) signals in the liver, the types of subcellular, cellular, and intercellular signals that occur, the physiological role of Ca(2+) signaling in the liver, and the role of Ca(2+) signaling in liver disease.
Figures
Similar articles
-
Calcium Signaling in Cholangiocytes: Methods, Mechanisms, and Effects.Int J Mol Sci. 2018 Dec 6;19(12):3913. doi: 10.3390/ijms19123913. Int J Mol Sci. 2018. PMID: 30563259 Free PMC article. Review.
-
Intercellular calcium waves integrate hormonal control of glucose output in the intact liver.J Physiol. 2019 Jun;597(11):2867-2885. doi: 10.1113/JP277650. Epub 2019 Apr 29. J Physiol. 2019. PMID: 30968953 Free PMC article.
-
The basis of nuclear phospholipase C in cell proliferation.Adv Biol Regul. 2021 Dec;82:100834. doi: 10.1016/j.jbior.2021.100834. Epub 2021 Oct 23. Adv Biol Regul. 2021. PMID: 34710785 Free PMC article. Review.
-
Fundamentals of Cellular Calcium Signaling: A Primer.Cold Spring Harb Perspect Biol. 2020 Jan 2;12(1):a038802. doi: 10.1101/cshperspect.a038802. Cold Spring Harb Perspect Biol. 2020. PMID: 31427372 Free PMC article. Review.
-
Calcium signaling in the nucleus.Can J Physiol Pharmacol. 2006 Mar-Apr;84(3-4):325-32. doi: 10.1139/y05-117. Can J Physiol Pharmacol. 2006. PMID: 16902580 Review.
Cited by
-
Involvement of oxidative species in cyclosporine-mediated cholestasis.Front Pharmacol. 2022 Nov 8;13:1004844. doi: 10.3389/fphar.2022.1004844. eCollection 2022. Front Pharmacol. 2022. PMID: 36425570 Free PMC article. Review.
-
Causality Analysis and Cell Network Modeling of Spatial Calcium Signaling Patterns in Liver Lobules.Front Physiol. 2018 Oct 4;9:1377. doi: 10.3389/fphys.2018.01377. eCollection 2018. Front Physiol. 2018. PMID: 30337879 Free PMC article.
-
Cypermethrin induces Sertoli cell apoptosis through mitochondrial pathway associated with calcium.Toxicol Res (Camb). 2021 Jun 19;10(4):742-750. doi: 10.1093/toxres/tfab056. eCollection 2021 Aug. Toxicol Res (Camb). 2021. PMID: 34484665 Free PMC article.
-
A Spatial Model of Hepatic Calcium Signaling and Glucose Metabolism Under Autonomic Control Reveals Functional Consequences of Varying Liver Innervation Patterns Across Species.Front Physiol. 2021 Nov 26;12:748962. doi: 10.3389/fphys.2021.748962. eCollection 2021. Front Physiol. 2021. PMID: 34899380 Free PMC article.
-
PDE2A Is Indispensable for Mouse Liver Development and Hematopoiesis.Int J Mol Sci. 2020 Apr 21;21(8):2902. doi: 10.3390/ijms21082902. Int J Mol Sci. 2020. PMID: 32326334 Free PMC article.
References
-
- Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1326. - PubMed
-
- Alonso MT, Garcia-Sancho J. Nuclear Ca(2+) signalling. Cell Calcium. 2011;49:280–289. - PubMed
-
- Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, Lesage G, Larusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767–G775. - PubMed
-
- Alpini G, Lenzi R, Zhai WR, Slott PA, Liu MH, Sarkozi L, Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol. 1989;257:G124–G133. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

