Deficient FGF signaling causes optic nerve dysgenesis and ocular coloboma
- PMID: 23720040
- PMCID: PMC3678341
- DOI: 10.1242/dev.089987
Deficient FGF signaling causes optic nerve dysgenesis and ocular coloboma
Abstract
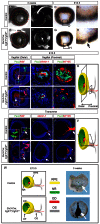
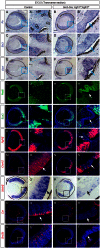
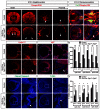
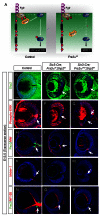
FGF signaling plays a pivotal role in eye development. Previous studies using in vitro chick models and systemic zebrafish mutants have suggested that FGF signaling is required for the patterning and specification of the optic vesicle, but due to a lack of genetic models, its role in mammalian retinal development remains elusive. In this study, we show that specific deletion of Fgfr1 and Fgfr2 in the optic vesicle disrupts ERK signaling, which results in optic disc and nerve dysgenesis and, ultimately, ocular coloboma. Defective FGF signaling does not abrogate Shh or BMP signaling, nor does it affect axial patterning of the optic vesicle. Instead, FGF signaling regulates Mitf and Pax2 in coordinating the closure of the optic fissure and optic disc specification, which is necessary for the outgrowth of the optic nerve. Genetic evidence further supports that the formation of an Frs2α-Shp2 complex and its recruitment to FGF receptors are crucial for downstream ERK signaling in this process, whereas constitutively active Ras signaling can rescue ocular coloboma in the FGF signaling mutants. Our results thus reveal a previously unappreciated role of FGF-Frs2α-Shp2-Ras-ERK signaling axis in preventing ocular coloboma. These findings suggest that components of FGF signaling pathway may be novel targets in the diagnosis of and the therapeutic interventions for congenital ocular anomalies.
Keywords: Coloboma; FGF; Frs2α; Mitf; Mouse; Optic disc; Optic fissure; Optic nerve; Pax2; Ras; Shp2.
Figures


Similar articles
-
Defective FGF signaling causes coloboma formation and disrupts retinal neurogenesis.Cell Res. 2013 Feb;23(2):254-73. doi: 10.1038/cr.2012.150. Epub 2012 Nov 13. Cell Res. 2013. PMID: 23147794 Free PMC article.
-
Frs2α and Shp2 signal independently of Gab to mediate FGF signaling in lens development.J Cell Sci. 2014 Feb 1;127(Pt 3):571-82. doi: 10.1242/jcs.134478. Epub 2013 Nov 27. J Cell Sci. 2014. PMID: 24284065 Free PMC article.
-
Fibroblast growth factor receptor 1 (FGFR1) tyrosine phosphorylation regulates binding of FGFR substrate 2alpha (FRS2alpha) but not FRS2 to the receptor.Mol Endocrinol. 2008 Jan;22(1):167-75. doi: 10.1210/me.2007-0140. Epub 2007 Sep 27. Mol Endocrinol. 2008. PMID: 17901128 Free PMC article.
-
Genes and pathways in optic fissure closure.Semin Cell Dev Biol. 2019 Jul;91:55-65. doi: 10.1016/j.semcdb.2017.10.010. Epub 2017 Dec 6. Semin Cell Dev Biol. 2019. PMID: 29198497 Review.
-
Review of evidence for environmental causes of uveal coloboma.Surv Ophthalmol. 2022 Jul-Aug;67(4):1031-1047. doi: 10.1016/j.survophthal.2021.12.008. Epub 2021 Dec 31. Surv Ophthalmol. 2022. PMID: 34979194 Free PMC article. Review.
Cited by
-
Nf2 fine-tunes proliferation and tissue alignment during closure of the optic fissure in the embryonic mouse eye.Hum Mol Genet. 2020 Dec 18;29(20):3373-3387. doi: 10.1093/hmg/ddaa228. Hum Mol Genet. 2020. PMID: 33075808 Free PMC article.
-
Development of astrocytes in the vertebrate eye.Dev Dyn. 2014 Dec;243(12):1501-10. doi: 10.1002/dvdy.24190. Epub 2014 Oct 13. Dev Dyn. 2014. PMID: 25236977 Free PMC article.
-
Multiple roles for Pax2 in the embryonic mouse eye.Dev Biol. 2021 Apr;472:18-29. doi: 10.1016/j.ydbio.2020.12.020. Epub 2021 Jan 9. Dev Biol. 2021. PMID: 33428890 Free PMC article.
-
Signaling - transcription interactions in mouse retinal ganglion cells early axon pathfinding -a literature review.Front Ophthalmol (Lausanne). 2023 May 17;3:1180142. doi: 10.3389/fopht.2023.1180142. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983012 Free PMC article. Review.
-
Meis homeobox genes control progenitor competence in the retina.Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2013136118. doi: 10.1073/pnas.2013136118. Proc Natl Acad Sci U S A. 2021. PMID: 33723039 Free PMC article.
References
-
- Ascaso F. J., Del Buey M. A., Huerva V., Latre B., Palomar A. (1993). Noonan’s syndrome with keratoconus and optic disc coloboma. Eur. J. Ophthalmol. 3, 101–103 - PubMed
-
- Barbieri A. M., Broccoli V., Bovolenta P., Alfano G., Marchitiello A., Mocchetti C., Crippa L., Bulfone A., Marigo V., Ballabio A., et al. (2002). Vax2 inactivation in mouse determines alteration of the eye dorsal-ventral axis, misrouting of the optic fibres and eye coloboma. Development 129, 805–813 - PubMed
-
- Bäumer N., Marquardt T., Stoykova A., Spieler D., Treichel D., Ashery-Padan R., Gruss P. (2003). Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development 130, 2903–2915 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

