Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model
- PMID: 23719296
- PMCID: PMC3709653
- DOI: 10.1182/blood-2013-01-482224
Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model
Abstract
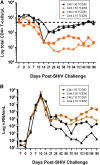
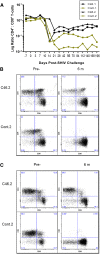
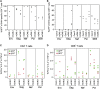
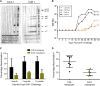
Despite continued progress in the development of novel antiretroviral therapies, it has become increasingly evident that drug-based treatments will not lead to a functional or sterilizing cure for HIV(+) patients. In 2009, an HIV(+) patient was effectively cured of HIV following allogeneic transplantation of hematopoietic stem cells (HSCs) from a CCR5(-/-) donor. The utility of this approach, however, is severely limited because of the difficulty in finding matched donors. Hence, we studied the potential of HIV-resistant stem cells in the autologous setting in a nonhuman primate AIDS model and incorporated a fusion inhibitor (mC46) as the means for developing infection-resistant cells. Pigtail macaques underwent identical transplants and Simian-Human Immunodeficiency Virus (SHIV) challenge procedures with the only variation between control and mC46 macaques being the inclusion of a fusion-inhibitor expression cassette. Following SHIV challenge, mC46 macaques, but not control macaques, showed a positive selection of gene-modified CD4(+) T cells in peripheral blood, gastrointestinal tract, and lymph nodes, accounting for >90% of the total CD4(+) T-cell population. mC46 macaques also maintained high frequencies of SHIV-specific, gene-modified CD4(+) T cells, an increase in nonmodified CD4(+) T cells, enhanced cytotoxic T lymphocyte function, and antibody responses. These data suggest that HSC protection may be a potential alternative to conventional antiretroviral therapy in patients with HIV/AIDS.
Figures

Similar articles
-
Lentivirus-mediated Gene Transfer in Hematopoietic Stem Cells Is Impaired in SHIV-infected, ART-treated Nonhuman Primates.Mol Ther. 2015 May;23(5):943-951. doi: 10.1038/mt.2015.19. Epub 2015 Feb 4. Mol Ther. 2015. PMID: 25648264 Free PMC article.
-
Pathogenic infection of Macaca nemestrina with a CCR5-tropic subtype-C simian-human immunodeficiency virus.Retrovirology. 2009 Jul 14;6:65. doi: 10.1186/1742-4690-6-65. Retrovirology. 2009. PMID: 19602283 Free PMC article.
-
Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model.AIDS Res Hum Retroviruses. 2006 Jun;22(6):580-8. doi: 10.1089/aid.2006.22.580. AIDS Res Hum Retroviruses. 2006. PMID: 16796533
-
Genetically modified hematopoietic stem cell transplantation for HIV-1-infected patients: can we achieve a cure?Mol Ther. 2014 Feb;22(2):257-264. doi: 10.1038/mt.2013.264. Epub 2013 Nov 13. Mol Ther. 2014. PMID: 24220323 Free PMC article. Review.
-
Evolution and Diversity of Immune Responses during Acute HIV Infection.Immunity. 2020 Nov 17;53(5):908-924. doi: 10.1016/j.immuni.2020.10.015. Immunity. 2020. PMID: 33207216 Free PMC article. Review.
Cited by
-
Engineering CAR T Cells to Target the HIV Reservoir.Front Cell Infect Microbiol. 2020 Aug 13;10:410. doi: 10.3389/fcimb.2020.00410. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32903563 Free PMC article. Review.
-
Stem cell gene therapy for HIV: strategies to inhibit viral entry and replication.Curr HIV/AIDS Rep. 2015 Mar;12(1):79-87. doi: 10.1007/s11904-014-0242-8. Curr HIV/AIDS Rep. 2015. PMID: 25578054 Review.
-
A novel real-time CTL assay to measure designer T-cell function against HIV Env(+) cells.J Med Primatol. 2014 Oct;43(5):341-8. doi: 10.1111/jmp.12137. Epub 2014 Aug 20. J Med Primatol. 2014. PMID: 25138734 Free PMC article.
-
A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates.Sci Transl Med. 2017 Nov 1;9(414):eaan1145. doi: 10.1126/scitranslmed.aan1145. Sci Transl Med. 2017. PMID: 29093179 Free PMC article.
-
Potent dual block to HIV-1 infection using lentiviral vectors expressing fusion inhibitor peptide mC46- and Vif-resistant APOBEC3G.Mol Ther Nucleic Acids. 2023 Aug 11;33:794-809. doi: 10.1016/j.omtn.2023.08.007. eCollection 2023 Sep 12. Mol Ther Nucleic Acids. 2023. PMID: 37662965 Free PMC article.
References
-
- Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292(2):251–265. - PubMed
-
- Carr A. Toxicity of antiretroviral therapy and implications for drug development [Review]. Nat Rev Drug Discov. 2003;2(8):624–634. - PubMed
-
- Louie M, Hogan C, Di Mascio M, et al. Determining the relative efficacy of highly active antiretroviral therapy. J Infect Dis. 2003;187(6):896–900. - PubMed
-
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277(5322):112–116. - PubMed
-
- Autran B. Strategies toward restoration of immunity to HIV. AIDS. 2002;16(10):4–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

