Therapeutic options in docetaxel-refractory metastatic castration-resistant prostate cancer: a cost-effectiveness analysis
- PMID: 23717582
- PMCID: PMC3661482
- DOI: 10.1371/journal.pone.0064275
Therapeutic options in docetaxel-refractory metastatic castration-resistant prostate cancer: a cost-effectiveness analysis
Abstract
Background: Docetaxel is an established first-line therapy to treat metastatic castration-resistant prostate cancer (mCRPC). Recently, abiraterone and cabazitaxel were approved for use after docetaxel failure, with improved survival. National Institute for Health and Clinical Excellence (NICE) preliminary recommendations were negative for both abiraterone (now positive in final recommendation) and cabazitaxel (negative in final recommendation).
Objective: To evaluate the cost-effectiveness of abiraterone, cabazitaxel, mitoxantrone and prednisone for mCRPC treatment in US.
Methods: A decision-tree model was constructed to compare the two mCRPC treatments versus two placebos over 18 months from a societal perspective. Chance nodes include baseline pain as a severity indicator, grade III/IV side-effects, and survival at 18 months. Probabilities, survival and health utilities were from published studies. Model cost inputs included drug treatment, side-effect management and prevention, radiation for pain, and death associated costs in 2010 US dollars.
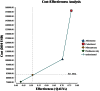
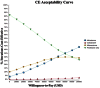
Results: Abiraterone is a cost-effective choice at $94K/QALY (quality adjusted life years) compared to placebo in our base-case analysis. Cabazitaxel and abiraterone are the most effective, yet also most expensive agents. The incremental cost-effectiveness ratios (ICER) at base-case are $101K/QALY (extended dominated) for mitoxantrone vs. placebo, $91K/QALY for abiraterone vs. mitoxantrone, $956K/QALY for cabazitaxel vs. abiraterone. Abiraterone becomes less cost-effective as its AWP increases, or if the cost of mitoxantrone side-effect management decreases. Increases in the percentage of patients with baseline pain leads to an increased ICER for both mitoxantrone and abiraterone, but mitoxantrone does relatively better. Cabazitaxel remains not cost-effective.
Conclusion: Our base case model suggests that abiraterone is a cost-effective option in docetaxel-refractory mCRPC patients. Newer treatments will also need a CEA assessment compared to abiraterone.
Conflict of interest statement
Figures

Similar articles
-
Cost-effectiveness analysis of cabazitaxel for metastatic castration resistant prostate cancer after docetaxel and androgen-signaling-targeted inhibitor resistance.BMC Cancer. 2021 Jan 7;21(1):35. doi: 10.1186/s12885-020-07754-9. BMC Cancer. 2021. PMID: 33413230 Free PMC article.
-
Cabazitaxel for Hormone-Relapsed Metastatic Prostate Cancer Previously Treated With a Docetaxel-Containing Regimen: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2017 Apr;35(4):415-424. doi: 10.1007/s40273-016-0457-1. Pharmacoeconomics. 2017. PMID: 27770303 Review.
-
Cost-effectiveness model of abiraterone plus prednisone, cabazitaxel plus prednisone and enzalutamide for visceral metastatic castration resistant prostate cancer therapy after docetaxel therapy resistance.J Med Econ. 2019 Nov;22(11):1202-1209. doi: 10.1080/13696998.2019.1661581. Epub 2019 Sep 17. J Med Econ. 2019. PMID: 31452414
-
New therapeutic options in metastatic castration-resistant prostate cancer: Can cost-effectiveness analysis help in treatment decisions?J Oncol Pharm Pract. 2014 Dec;20(6):417-25. doi: 10.1177/1078155213509505. Epub 2013 Nov 14. J Oncol Pharm Pract. 2014. PMID: 24243919
-
Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer.Drug Des Devel Ther. 2011 Mar 10;5:117-24. doi: 10.2147/DDDT.S13029. Drug Des Devel Ther. 2011. PMID: 21448449 Free PMC article. Review.
Cited by
-
Cost-effectiveness analyses and cost analyses in castration-resistant prostate cancer: A systematic review.PLoS One. 2018 Dec 5;13(12):e0208063. doi: 10.1371/journal.pone.0208063. eCollection 2018. PLoS One. 2018. PMID: 30517165 Free PMC article.
-
Cost-effectiveness analysis of cabazitaxel for metastatic castration resistant prostate cancer after docetaxel and androgen-signaling-targeted inhibitor resistance.BMC Cancer. 2021 Jan 7;21(1):35. doi: 10.1186/s12885-020-07754-9. BMC Cancer. 2021. PMID: 33413230 Free PMC article.
-
Treatment Sequences and Pharmacy Costs of 2 New Therapies for Metastatic Castration-Resistant Prostate Cancer.Am Health Drug Benefits. 2015 Jun;8(4):185-95. Am Health Drug Benefits. 2015. PMID: 26157540 Free PMC article.
-
Cost-effectiveness analysis of 7 treatments in metastatic hormone-sensitive prostate cancer: a public-payer perspective.J Natl Cancer Inst. 2023 Nov 8;115(11):1374-1382. doi: 10.1093/jnci/djad135. J Natl Cancer Inst. 2023. PMID: 37436697 Free PMC article.
-
Emerging perspectives in prostate cancer: Insights from the 4th Asia Pacific Prostate Cancer Conference.Prostate Int. 2015 Dec;3(Suppl):S1-4. doi: 10.1016/j.prnil.2015.10.014. Epub 2015 Oct 22. Prostate Int. 2015. PMID: 26858943 Free PMC article. No abstract available.
References
-
- U.S. Cancer Statistics Working Group (2010) United States Cancer Statistics: 1999–2007 Cancer Incidence and Mortatlity web-based Report. Available: http://appsnccdcdcgov/uscs/. Atlanta, GA: Accessed 2012 March.
-
- Jermal A, Siegel R, Xu J, Ward E (2010) Cancer Statistics. CA Cancer J Clin 60: 277–300. - PubMed
-
- National Comprehensive Cancer Network Physician Guidelines Working Group (2011) NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer–V.4.2011. Available: http://wwwnccnorg/professionals/physician_gls/f_guidelinesasp Accessed 2012 Feb 21.
-
- Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, et al. (2008) Survival and PSA response of patients in the TAX 327 study who crossed over to receive docetaxel after mitoxantrone or vice versa. AnnOnco l19: 1749–1753. - PubMed
-
- Petrylak DP, Tangen CM, Hussaine MH, Lara PN Jr, Jones JA, et al. (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Eng J Med 351: 1513–1520. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

