To breathe or not to breathe: the haematopoietic stem/progenitor cells dilemma
- PMID: 23714011
- PMCID: PMC3753828
- DOI: 10.1111/bph.12253
To breathe or not to breathe: the haematopoietic stem/progenitor cells dilemma
Abstract
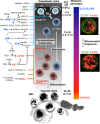
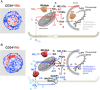
Adult haematopoietic stem/progenitor cells (HSPCs) constitute the lifespan reserve for the generation of all the cellular lineages in the blood. Although massive progress in identifying the cluster of master genes controlling self-renewal and multipotency has been achieved in the past decade, some aspects of the physiology of HSPCs still need to be clarified. In particular, there is growing interest in the metabolic profile of HSPCs in view of their emerging role as determinants of cell fate. Indeed, stem cells and progenitors have distinct metabolic profiles, and the transition from stem to progenitor cell corresponds to a critical metabolic change, from glycolysis to oxidative phosphorylation. In this review, we summarize evidence, reported in the literature and provided by our group, highlighting the peculiar ability of HSPCs to adapt their mitochondrial oxidative/bioenergetic metabolism to survive in the hypoxic microenvironment of the endoblastic niche and to exploit redox signalling in controlling the balance between quiescence versus active cycling and differentiation. Especial prominence is given to the interplay between hypoxia inducible factor-1, globins and NADPH oxidases in managing the mitochondrial dioxygen-related metabolism and biogenesis in HSPCs under different ambient conditions. A mechanistic model is proposed whereby 'mitochondrial differentiation' is a prerequisite in uncommitted stem cells, paving the way for growth/differentiation factor-dependent processes. Advancing the understanding of stem cell metabolism will, hopefully, help to (i) improve efforts to maintain, expand and manipulate HSPCs ex vivo and realize their potential therapeutic benefits in regenerative medicine; (ii) reprogramme somatic cells to generate stem cells; and (iii) eliminate, selectively, malignant stem cells.
Linked articles: This article is part of a themed section on Emerging Therapeutic Aspects in Oncology. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2013.169.issue-8.
Keywords: NADPH oxidases; globins; haematopoietic stem/progenitor cells; hypoxia inducible factor-1; mitochondria; oxidative phosphorylation; redox signalling.
© 2013 The British Pharmacological Society.
Figures
Similar articles
-
Role of reactive oxygen species as signal molecules in the pre-commitment phase of adult stem cells.Ital J Biochem. 2007 Dec;56(4):295-301. Ital J Biochem. 2007. PMID: 19192630 Review.
-
Connecting Mitochondria, Metabolism, and Stem Cell Fate.Stem Cells Dev. 2015 Sep 1;24(17):1957-71. doi: 10.1089/scd.2015.0117. Epub 2015 Jul 2. Stem Cells Dev. 2015. PMID: 26134242 Free PMC article. Review.
-
Oxidative stress and hypoxia in normal and leukemic stem cells.Exp Hematol. 2016 Jul;44(7):540-60. doi: 10.1016/j.exphem.2016.04.012. Epub 2016 May 12. Exp Hematol. 2016. PMID: 27179622 Review.
-
Electron transport chain complex II sustains high mitochondrial membrane potential in hematopoietic stem and progenitor cells.Stem Cell Res. 2019 Oct;40:101573. doi: 10.1016/j.scr.2019.101573. Epub 2019 Sep 10. Stem Cell Res. 2019. PMID: 31539857 Free PMC article.
-
Analysis of Hematopoietic Stem Progenitor Cell Metabolism.J Vis Exp. 2019 Nov 9;(153):10.3791/60234. doi: 10.3791/60234. J Vis Exp. 2019. PMID: 31762453 Free PMC article.
Cited by
-
Divide and conquer: two stem cell populations in squamous epithelia, reserves and the active duty forces.Int J Oral Sci. 2019 Aug 27;11(3):26. doi: 10.1038/s41368-019-0061-2. Int J Oral Sci. 2019. PMID: 31451683 Free PMC article. Review.
-
Emerging therapeutic aspects in oncology.Br J Pharmacol. 2013 Aug;169(8):1647-51. doi: 10.1111/bph.12304. Br J Pharmacol. 2013. PMID: 23889318 Free PMC article.
-
Hematopoietic stem cell fate through metabolic control.Exp Hematol. 2018 Aug;64:1-11. doi: 10.1016/j.exphem.2018.05.005. Epub 2018 May 25. Exp Hematol. 2018. PMID: 29807063 Free PMC article. Review.
-
Metabolism and the Control of Cell Fate Decisions and Stem Cell Renewal.Annu Rev Cell Dev Biol. 2016 Oct 6;32:399-409. doi: 10.1146/annurev-cellbio-111315-125134. Epub 2016 Aug 1. Annu Rev Cell Dev Biol. 2016. PMID: 27482603 Free PMC article. Review.
-
Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity.Sci Rep. 2020 Feb 10;10(1):2287. doi: 10.1038/s41598-020-58871-1. Sci Rep. 2020. PMID: 32041983 Free PMC article.
References
-
- Baldwin MA, Benz CC. Redox control of zinc finger proteins. Methods Enzymol. 2002;353:54–69. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

