SERCA2a: a prime target for modulation of cardiac contractility during heart failure
- PMID: 23710633
- PMCID: PMC4133892
- DOI: 10.5483/bmbrep.2013.46.5.077
SERCA2a: a prime target for modulation of cardiac contractility during heart failure
Abstract
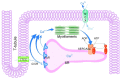
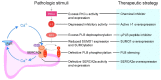
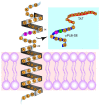
Heart failure is one of the leading causes of sudden death in developed countries. While current therapies are mostly aimed at mitigating associated symptoms, novel therapies targeting the subcellular mechanisms underlying heart failure are emerging. Failing hearts are characterized by reduced contractile properties caused by impaired Ca(2+) cycling between the sarcoplasm and sarcoplasmic reticulum (SR). Sarcoplasmic/ endoplasmic reticulum Ca(2+)ATPase 2a (SERCA2a) mediates Ca(2+) reuptake into the SR in cardiomyocytes. Of note, the expression level and/or activity of SERCA2a, translating to the quantity of SR Ca(2+) uptake, are significantly reduced in failing hearts. Normalization of the SERCA2a expression level by gene delivery has been shown to restore hampered cardiac functions and ameliorate associated symptoms in pre-clinical as well as clinical studies. SERCA2a activity can be regulated at multiple levels of a signaling cascade comprised of phospholamban, protein phosphatase 1, inhibitor-1, and PKCα. SERCA2 activity is also regulated by post-translational modifications including SUMOylation and acetylation. In this review, we will highlight the molecular mechanisms underlying the regulation of SERCA2a activity and the potential therapeutic modalities for the treatment of heart failure.
Figures
Similar articles
-
SERCA2a: a key protein in the Ca2+ cycle of the heart failure.Heart Fail Rev. 2020 May;25(3):523-535. doi: 10.1007/s10741-019-09873-3. Heart Fail Rev. 2020. PMID: 31701344 Review.
-
Istaroxime stimulates SERCA2a and accelerates calcium cycling in heart failure by relieving phospholamban inhibition.Br J Pharmacol. 2013 Aug;169(8):1849-61. doi: 10.1111/bph.12278. Br J Pharmacol. 2013. PMID: 23763364 Free PMC article.
-
[Modification of sarco-endoplasmic reticulum Ca(2 +) -ATPase in the failing cardiomyocyte].Clin Calcium. 2013 Apr;23(4):535-42. Clin Calcium. 2013. PMID: 23545743 Review. Japanese.
-
Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation.Cardiovasc Res. 2003 Jan;57(1):20-7. doi: 10.1016/s0008-6363(02)00694-6. Cardiovasc Res. 2003. PMID: 12504810 Review.
-
Role of SIRT1 in Modulating Acetylation of the Sarco-Endoplasmic Reticulum Ca2+-ATPase in Heart Failure.Circ Res. 2019 Apr 26;124(9):e63-e80. doi: 10.1161/CIRCRESAHA.118.313865. Circ Res. 2019. PMID: 30786847 Free PMC article.
Cited by
-
Luteolin improves cardiac dysfunction in heart failure rats by regulating sarcoplasmic reticulum Ca2+-ATPase 2a.Sci Rep. 2017 Jan 23;7:41017. doi: 10.1038/srep41017. Sci Rep. 2017. PMID: 28112209 Free PMC article.
-
The Protective Effect of Qishen Granule on Heart Failure after Myocardial Infarction through Regulation of Calcium Homeostasis.Evid Based Complement Alternat Med. 2020 Oct 22;2020:1868974. doi: 10.1155/2020/1868974. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33149749 Free PMC article.
-
Recovery of the failing heart: emerging approaches and mechanisms in excitation-contraction coupling.F1000Prime Rep. 2014 May 6;6:27. doi: 10.12703/P6-27. eCollection 2014. F1000Prime Rep. 2014. PMID: 24904750 Free PMC article. Review.
-
Enhancing myocardial function with cardiac contractility modulation: potential and challenges.ESC Heart Fail. 2024 Feb;11(1):1-12. doi: 10.1002/ehf2.14575. Epub 2023 Nov 10. ESC Heart Fail. 2024. PMID: 37947013 Free PMC article. Review.
-
Structure-function relationships and modifications of cardiac sarcoplasmic reticulum Ca2+-transport.Physiol Res. 2021 Dec 30;70(Suppl4):S443-S470. doi: 10.33549/physiolres.934805. Physiol Res. 2021. PMID: 35199536 Free PMC article. Review.
References
-
- Braz J. C., Gregory K., Pathak A., Zhao W., Sahin B., Klevitsky R., Kimball T. F., Lorenz J. N., Nairn A. C., Liggett S. B., Bodi I., Wang S., Schwartz A., Lakatta E. G., DePaoli-Roach A. A., Robbins J., Hewett T. E., Bibb J. A., Westfall M. V., Kranias E. G., Molkentin J. D. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat. Med. (2004);10:248–254. doi: 10.1038/nm1000. - DOI - PubMed
-
- Carr A. N., Schmidt A. G., Suzuki Y., del Monte F., Sato Y., Lanner C., Breeden K., Jing S. L., Allen P. B., Greengard P., Yatani A., Hoit B. D., Grupp I. L., Hajjar R. J., DePaoli-Roach A. A., Kranias E. G. Type 1 phosphatase, a negative regulator of cardiac function. Mol. Cell. Biol. (2002);22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. - DOI - PMC - PubMed
-
- de lMonte F., Harding S. E., Schmidt U., Matsui T., Kang Z. B., Dec G. W., Gwathmey J. K., Rosenzweig A., Hajjar R. J. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. (1999);100:2308–2311. doi: 10.1161/01.CIR.100.23.2308. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

