The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6
- PMID: 23709225
- PMCID: PMC3711295
- DOI: 10.1074/jbc.M113.479337
The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6
Abstract
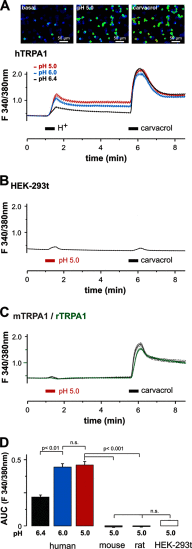
The surveillance of acid-base homeostasis is concerted by diverse mechanisms, including an activation of sensory afferents. Proton-evoked activation of rodent sensory neurons is mainly mediated by the capsaicin receptor TRPV1 and acid-sensing ion channels. In this study, we demonstrate that extracellular acidosis activates and sensitizes the human irritant receptor TRPA1 (hTRPA1). Proton-evoked membrane currents and calcium influx through hTRPA1 occurred at physiological acidic pH values, were concentration-dependent, and were blocked by the selective TRPA1 antagonist HC030031. Both rodent and rhesus monkey TRPA1 failed to respond to extracellular acidosis, and protons even inhibited rodent TRPA1. Accordingly, mouse dorsal root ganglion neurons lacking TRPV1 only responded to protons when hTRPA1 was expressed heterologously. This species-specific activation of hTRPA1 by protons was reversed in both mouse and rhesus monkey TRPA1 by exchange of distinct residues within transmembrane domains 5 and 6. Furthermore, protons seem to interact with an extracellular interaction site to gate TRPA1 and not via a modification of intracellular N-terminal cysteines known as important interaction sites for electrophilic TRPA1 agonists. Our data suggest that hTRPA1 acts as a sensor for extracellular acidosis in human sensory neurons and should thus be taken into account as a yet unrecognized transduction molecule for proton-evoked pain and inflammation. The species specificity of this property is unique among known endogenous TRPA1 agonists, possibly indicating that evolutionary pressure enforced TRPA1 to inherit the role as an acid sensor in human sensory neurons.
Keywords: Acid-sensing Ion Channels (ASIC); Acidosis; Nociceptor; Pain; Patch Clamp; Species Specificity; TRP Channels.
Figures


Similar articles
-
Primary alcohols activate human TRPA1 channel in a carbon chain length-dependent manner.Pflugers Arch. 2012 Apr;463(4):549-59. doi: 10.1007/s00424-011-1069-4. Epub 2012 Jan 6. Pflugers Arch. 2012. PMID: 22222967
-
Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception.Am J Physiol Cell Physiol. 2011 Sep;301(3):C587-600. doi: 10.1152/ajpcell.00465.2010. Epub 2011 Jun 8. Am J Physiol Cell Physiol. 2011. PMID: 21653898 Free PMC article.
-
The peptide Phα1β, from spider venom, acts as a TRPA1 channel antagonist with antinociceptive effects in mice.Br J Pharmacol. 2017 Jan;174(1):57-69. doi: 10.1111/bph.13652. Epub 2016 Nov 28. Br J Pharmacol. 2017. PMID: 27759880 Free PMC article.
-
[Activation and regulation of nociceptive transient receptor potential (TRP) channels, TRPV1 and TRPA1].Yakugaku Zasshi. 2010 Mar;130(3):289-94. doi: 10.1248/yakushi.130.289. Yakugaku Zasshi. 2010. PMID: 20190512 Review. Japanese.
-
Irritating channels: the case of TRPA1.J Physiol. 2011 Apr 1;589(Pt 7):1543-9. doi: 10.1113/jphysiol.2010.200717. Epub 2010 Nov 15. J Physiol. 2011. PMID: 21078588 Free PMC article. Review.
Cited by
-
Differential Activation of TRP Channels in the Adult Rat Spinal Substantia Gelatinosa by Stereoisomers of Plant-Derived Chemicals.Pharmaceuticals (Basel). 2016 Jul 28;9(3):46. doi: 10.3390/ph9030046. Pharmaceuticals (Basel). 2016. PMID: 27483289 Free PMC article. Review.
-
Non-Analgesic Symptomatic or Disease-Modifying Potential of TRPA1.Med Sci (Basel). 2019 Sep 23;7(10):99. doi: 10.3390/medsci7100099. Med Sci (Basel). 2019. PMID: 31547502 Free PMC article. Review.
-
Specific Attenuation of Purinergic Signaling during Bortezomib-Induced Peripheral Neuropathy In Vitro.Int J Mol Sci. 2022 Mar 29;23(7):3734. doi: 10.3390/ijms23073734. Int J Mol Sci. 2022. PMID: 35409095 Free PMC article.
-
Emerging Perspectives on Pain Management by Modulation of TRP Channels and ANO1.Int J Mol Sci. 2019 Jul 11;20(14):3411. doi: 10.3390/ijms20143411. Int J Mol Sci. 2019. PMID: 31336748 Free PMC article. Review.
-
Transient Receptor Potential Channel A1 (TRPA1) Regulates Sulfur Mustard-Induced Expression of Heat Shock 70 kDa Protein 6 (HSPA6) In Vitro.Cells. 2018 Aug 31;7(9):126. doi: 10.3390/cells7090126. Cells. 2018. PMID: 30200301 Free PMC article.
References
-
- Reeh P. W., Kress M. (2001) Molecular physiology of proton transduction in nociceptors. Curr. Opin. Pharmacol 1, 45–51 - PubMed
-
- Krishtal O. A., Pidoplichko V. I. (1981) Receptor for protons in the membrane of sensory neurons. Brain Res. 214, 150–154 - PubMed
-
- Deval E., Gasull X., Noël J., Salinas M., Baron A., Diochot S., Lingueglia E. (2010) Acid-sensing ion channels (ASICs). Pharmacology and implication in pain. Pharmacol. Ther. 128, 549–558 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

