Disconnecting mitochondrial content from respiratory chain capacity in PGC-1-deficient skeletal muscle
- PMID: 23707060
- PMCID: PMC3688451
- DOI: 10.1016/j.celrep.2013.04.023
Disconnecting mitochondrial content from respiratory chain capacity in PGC-1-deficient skeletal muscle
Abstract
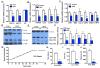
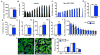
The transcriptional coactivators PGC-1α and PGC-1β are widely thought to be required for mitochondrial biogenesis and fiber typing in skeletal muscle. Here, we show that mice lacking both PGC-1s in myocytes do indeed have profoundly deficient mitochondrial respiration but, surprisingly, have preserved mitochondrial content, isolated muscle contraction capacity, fiber-type composition, in-cage ambulation, and voluntary running capacity. Most of these findings are recapitulated in cell culture and, thus, are cell autonomous. Functional electron microscopy reveals normal cristae density with decreased cytochrome oxidase activity. These data lead to the following surprising conclusions: (1) PGC-1s are in fact dispensable for baseline muscle function, mitochondrial content, and fiber typing, (2) endurance fatigue at low workloads is not limited by muscle mitochondrial capacity, and (3) mitochondrial content and cristae density can be dissociated from respiratory capacity.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle.Am J Physiol Cell Physiol. 2010 Mar;298(3):C572-9. doi: 10.1152/ajpcell.00481.2009. Epub 2009 Dec 23. Am J Physiol Cell Physiol. 2010. PMID: 20032509 Free PMC article.
-
The role of PGC-1alpha on mitochondrial function and apoptotic susceptibility in muscle.Am J Physiol Cell Physiol. 2009 Jul;297(1):C217-25. doi: 10.1152/ajpcell.00070.2009. Epub 2009 May 13. Am J Physiol Cell Physiol. 2009. PMID: 19439529
-
Adult skeletal muscle peroxisome proliferator-activated receptor γ -related coactivator 1 is involved in maintaining mitochondrial content.Am J Physiol Regul Integr Comp Physiol. 2023 Apr 1;324(4):R470-R479. doi: 10.1152/ajpregu.00241.2022. Epub 2023 Jan 30. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 36717166 Free PMC article.
-
Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis.Appl Physiol Nutr Metab. 2011 Oct;36(5):589-97. doi: 10.1139/h11-070. Epub 2011 Sep 2. Appl Physiol Nutr Metab. 2011. PMID: 21888529 Review.
-
Nitric oxide in skeletal muscle: role on mitochondrial biogenesis and function.Int J Mol Sci. 2012 Dec 14;13(12):17160-84. doi: 10.3390/ijms131217160. Int J Mol Sci. 2012. PMID: 23242154 Free PMC article. Review.
Cited by
-
Principles of Exercise Prescription, and How They Influence Exercise-Induced Changes of Transcription Factors and Other Regulators of Mitochondrial Biogenesis.Sports Med. 2018 Jul;48(7):1541-1559. doi: 10.1007/s40279-018-0894-4. Sports Med. 2018. PMID: 29675670 Review.
-
Contraction stimulates muscle glucose uptake independent of atypical PKC.Physiol Rep. 2015 Nov;3(11):e12565. doi: 10.14814/phy2.12565. Physiol Rep. 2015. PMID: 26564060 Free PMC article.
-
Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism.Nat Commun. 2015 Aug 24;6:8054. doi: 10.1038/ncomms9054. Nat Commun. 2015. PMID: 26299309 Free PMC article.
-
Autophagy-associated atrophy and metabolic remodeling of the mouse diaphragm after short-term intermittent hypoxia.PLoS One. 2015 Jun 24;10(6):e0131068. doi: 10.1371/journal.pone.0131068. eCollection 2015. PLoS One. 2015. PMID: 26107816 Free PMC article.
-
Sarcolipin Signaling Promotes Mitochondrial Biogenesis and Oxidative Metabolism in Skeletal Muscle.Cell Rep. 2018 Sep 11;24(11):2919-2931. doi: 10.1016/j.celrep.2018.08.036. Cell Rep. 2018. PMID: 30208317 Free PMC article.
References
-
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. - PubMed
-
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46. - PubMed
-
- Benit P, Goncalves S, Philippe Dassa E, Briere JJ, Martin G, Rustin P. Three spectrophotometric assays for the measurement of the five respiratory chain complexes in minuscule biological samples. Clin Chim Acta. 2006;374:81–86. - PubMed
-
- Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPAR{gamma} coactivator-1{alpha} improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

