The ATPase of the phi29 DNA packaging motor is a member of the hexameric AAA+ superfamily
- PMID: 23706809
- PMCID: PMC3700617
- DOI: 10.1016/j.virol.2013.04.004
The ATPase of the phi29 DNA packaging motor is a member of the hexameric AAA+ superfamily
Abstract
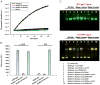
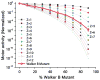
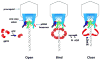
The AAA+ superfamily of proteins is a class of motor ATPases performing a wide range of functions that typically exist as hexamers. The ATPase of phi29 DNA packaging motor has long been a subject of debate in terms of stoichiometry and mechanism of action. Here, we confirmed the stoichiometry of phi29 motor ATPase to be a hexamer and provide data suggesting that the phi29 motor ATPase is a member of the classical hexameric AAA+ superfamily. Native PAGE, EMSA, capillary electrophoresis, ATP titration, and binomial distribution assay show that the ATPase is a hexamer. Mutations in the known Walker motifs of the ATPase validated our previous assumptions that the protein exists as another member of this AAA+ superfamily. Our data also supports the finding that the phi29 DNA packaging motor uses a revolution mechanism without rotation or coiling (Schwartz et al., this issue).
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


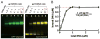
Similar articles
-
Mechanism of one-way traffic of hexameric phi29 DNA packaging motor with four electropositive relaying layers facilitating antiparallel revolution.ACS Nano. 2013 May 28;7(5):4082-92. doi: 10.1021/nn4002775. Epub 2013 Mar 26. ACS Nano. 2013. PMID: 23510192 Free PMC article.
-
Revolution rather than rotation of AAA+ hexameric phi29 nanomotor for viral dsDNA packaging without coiling.Virology. 2013 Aug 15;443(1):28-39. doi: 10.1016/j.virol.2013.04.019. Epub 2013 Jun 12. Virology. 2013. PMID: 23763768 Free PMC article.
-
An Arginine Finger Regulates the Sequential Action of Asymmetrical Hexameric ATPase in the Double-Stranded DNA Translocation Motor.Mol Cell Biol. 2016 Sep 12;36(19):2514-23. doi: 10.1128/MCB.00142-16. Print 2016 Oct 1. Mol Cell Biol. 2016. PMID: 27457616 Free PMC article.
-
Ultrastable pRNA hexameric ring gearing hexameric phi29 DNA-packaging motor by revolving without rotating and coiling.Curr Opin Biotechnol. 2013 Aug;24(4):581-90. doi: 10.1016/j.copbio.2013.03.019. Epub 2013 May 14. Curr Opin Biotechnol. 2013. PMID: 23683853 Free PMC article. Review.
-
"Push through one-way valve" mechanism of viral DNA packaging.Adv Virus Res. 2012;83:415-65. doi: 10.1016/B978-0-12-394438-2.00009-8. Adv Virus Res. 2012. PMID: 22748815 Review.
Cited by
-
Mechanism of one-way traffic of hexameric phi29 DNA packaging motor with four electropositive relaying layers facilitating antiparallel revolution.ACS Nano. 2013 May 28;7(5):4082-92. doi: 10.1021/nn4002775. Epub 2013 Mar 26. ACS Nano. 2013. PMID: 23510192 Free PMC article.
-
Finding of widespread viral and bacterial revolution dsDNA translocation motors distinct from rotation motors by channel chirality and size.Cell Biosci. 2014 Jun 1;4:30. doi: 10.1186/2045-3701-4-30. eCollection 2014. Cell Biosci. 2014. PMID: 24940480 Free PMC article.
-
Nanobiomotors of archaeal DNA repair machineries: current research status and application potential.Cell Biosci. 2014 Jun 25;4:32. doi: 10.1186/2045-3701-4-32. eCollection 2014. Cell Biosci. 2014. PMID: 24995126 Free PMC article. Review.
-
Elastic properties and heterogeneous stiffness of the phi29 motor connector channel.Biophys J. 2014 Mar 18;106(6):1338-48. doi: 10.1016/j.bpj.2014.01.028. Biophys J. 2014. PMID: 24655509 Free PMC article.
-
Engineered nanopore of Phi29 DNA-packaging motor for real-time detection of single colon cancer specific antibody in serum.ACS Nano. 2013 Nov 26;7(11):9814-22. doi: 10.1021/nn404435v. Epub 2013 Oct 30. ACS Nano. 2013. PMID: 24152066 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

