Transcriptional elongation checkpoint control in development and disease
- PMID: 23699407
- PMCID: PMC3672643
- DOI: 10.1101/gad.215137.113
Transcriptional elongation checkpoint control in development and disease
Abstract
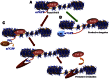
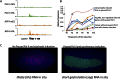
Transcriptional elongation control by RNA polymerase II and its associated factors has taken center stage as a process essential for the regulation of gene expression throughout development. In this review, we analyze recent findings on the identification of factors functioning in the regulation of the transcriptional elongation checkpoint control (TECC) stage of gene expression and how the factors' misregulation is associated with disease pathogenesis, including cancer.
Keywords: RNA polymerase II; cancer; chromatin; elongation; transcription.
Figures


Similar articles
-
The super elongation complex (SEC) family in transcriptional control.Nat Rev Mol Cell Biol. 2012 Sep;13(9):543-7. doi: 10.1038/nrm3417. Epub 2012 Aug 16. Nat Rev Mol Cell Biol. 2012. PMID: 22895430 Review.
-
Negative elongation factor (NELF) coordinates RNA polymerase II pausing, premature termination, and chromatin remodeling to regulate HIV transcription.J Biol Chem. 2013 Sep 6;288(36):25995-26003. doi: 10.1074/jbc.M113.496489. Epub 2013 Jul 24. J Biol Chem. 2013. PMID: 23884411 Free PMC article.
-
Sub1 associates with Spt5 and influences RNA polymerase II transcription elongation rate.Mol Biol Cell. 2012 Nov;23(21):4297-312. doi: 10.1091/mbc.E12-04-0331. Epub 2012 Sep 12. Mol Biol Cell. 2012. PMID: 22973055 Free PMC article.
-
An mRNA Capping Enzyme Targets FACT to the Active Gene To Enhance the Engagement of RNA Polymerase II into Transcriptional Elongation.Mol Cell Biol. 2017 Jun 15;37(13):e00029-17. doi: 10.1128/MCB.00029-17. Print 2017 Jul 1. Mol Cell Biol. 2017. PMID: 28396559 Free PMC article.
-
A role for histone acetylation in regulating transcription elongation.Transcription. 2018;9(4):225-232. doi: 10.1080/21541264.2017.1394423. Epub 2017 Dec 8. Transcription. 2018. PMID: 29219750 Free PMC article. Review.
Cited by
-
Stably paused genes revealed through inhibition of transcription initiation by the TFIIH inhibitor triptolide.Genes Dev. 2015 Jan 1;29(1):39-47. doi: 10.1101/gad.246173.114. Genes Dev. 2015. PMID: 25561494 Free PMC article.
-
Epigenetics of hematopoiesis and hematological malignancies.Genes Dev. 2016 Sep 15;30(18):2021-2041. doi: 10.1101/gad.284109.116. Genes Dev. 2016. PMID: 27798847 Free PMC article. Review.
-
Global unleashing of transcription elongation waves in response to genotoxic stress restricts somatic mutation rate.Nat Commun. 2017 Dec 12;8(1):2076. doi: 10.1038/s41467-017-02145-4. Nat Commun. 2017. PMID: 29233992 Free PMC article.
-
The plant RNA polymerase II elongation complex: A hub coordinating transcript elongation and mRNA processing.Transcription. 2018;9(2):117-122. doi: 10.1080/21541264.2017.1356902. Epub 2017 Oct 4. Transcription. 2018. PMID: 28886274 Free PMC article.
-
PAF1, a Molecular Regulator of Promoter-Proximal Pausing by RNA Polymerase II.Cell. 2015 Aug 27;162(5):1003-15. doi: 10.1016/j.cell.2015.07.042. Epub 2015 Aug 13. Cell. 2015. PMID: 26279188 Free PMC article.
References
-
- Aso T, Lane WS, Conaway JW, Conaway RC 1995. Elongin (SIII): A multisubunit regulator of elongation by RNA polymerase II. Science 269: 1439–1443 - PubMed
-
- Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM 2012. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem 287: 36609–36616 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
