Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR)
- PMID: 23696639
- PMCID: PMC3696645
- DOI: 10.1074/jbc.R113.479808
Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR)
Abstract
3-Hydroxy-3-methylglutaryl-CoA reductase (HMGCR) is the target of the statins, important drugs that lower blood cholesterol levels and treat cardiovascular disease. Consequently, the regulation of HMGCR has been investigated in detail. However, this enzyme acts very early in the cholesterol synthesis pathway, with ∼20 subsequent enzymes needed to produce cholesterol. How they are regulated is largely unexplored territory, but there is growing evidence that enzymes beyond HMGCR serve as flux-controlling points. Here, we introduce some of the known regulatory mechanisms affecting enzymes beyond HMGCR and highlight the need to further investigate their control.
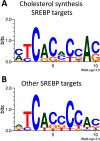
Keywords: Cholesterol; Cholesterol Regulation; ER-associated Degradation; NSDHL; Post-translational Modification; SREBP; Squalene Monooxygenase; Sterol.
Figures
Similar articles
-
Differential use of E2 ubiquitin conjugating enzymes for regulated degradation of the rate-limiting enzymes HMGCR and SQLE in cholesterol biosynthesis.Atherosclerosis. 2019 Feb;281:137-142. doi: 10.1016/j.atherosclerosis.2018.12.008. Epub 2018 Dec 23. Atherosclerosis. 2019. PMID: 30658189
-
Post-translational control of the long and winding road to cholesterol.J Biol Chem. 2020 Dec 18;295(51):17549-17559. doi: 10.1074/jbc.REV120.010723. J Biol Chem. 2020. PMID: 33453997 Free PMC article. Review.
-
Navigating the Shallows and Rapids of Cholesterol Synthesis Downstream of HMGCR.J Nutr Sci Vitaminol (Tokyo). 2015;61 Suppl:S154-6. doi: 10.3177/jnsv.61.S154. J Nutr Sci Vitaminol (Tokyo). 2015. PMID: 26598836 Review.
-
Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase.Cell Metab. 2011 Mar 2;13(3):260-73. doi: 10.1016/j.cmet.2011.01.015. Cell Metab. 2011. PMID: 21356516
-
Protein turnover regulated by cholesterol.Curr Opin Lipidol. 2012 Feb;23(1):76-7. doi: 10.1097/MOL.0b013e32834f42b3. Curr Opin Lipidol. 2012. PMID: 22241328 No abstract available.
Cited by
-
In Ovo injection of betaine affects hepatic cholesterol metabolism through epigenetic gene regulation in newly hatched chicks.PLoS One. 2015 Apr 10;10(4):e0122643. doi: 10.1371/journal.pone.0122643. eCollection 2015. PLoS One. 2015. PMID: 25860502 Free PMC article.
-
The role of noncoding RNAs in cancer lipid metabolism.Front Oncol. 2022 Nov 14;12:1026257. doi: 10.3389/fonc.2022.1026257. eCollection 2022. Front Oncol. 2022. PMID: 36452489 Free PMC article. Review.
-
Regulation of endoplasmic reticulum stress and trophectoderm lineage specification by the mevalonate pathway in the mouse preimplantation embryo.Mol Hum Reprod. 2021 Mar 24;27(4):gaab015. doi: 10.1093/molehr/gaab015. Mol Hum Reprod. 2021. PMID: 33677573 Free PMC article.
-
Improvement of hepatic bioavailability as a new step for the future of statin.Arch Med Sci. 2015 Apr 25;11(2):406-10. doi: 10.5114/aoms.2015.50972. Epub 2015 Apr 23. Arch Med Sci. 2015. PMID: 25995759 Free PMC article.
-
A variability in response of osteoclasts to zoledronic acid is mediated by smoking-associated modification in the DNA methylome.Clin Epigenetics. 2023 Mar 13;15(1):42. doi: 10.1186/s13148-023-01449-1. Clin Epigenetics. 2023. PMID: 36915112 Free PMC article.
References
-
- Schoenheimer R., Breusch F. (1933) Synthesis and destruction of cholesterol in the organism. J. Biol. Chem. 103, 439–448
-
- Bloch K. (1965) The biological synthesis of cholesterol. Science 150, 19–28 - PubMed
-
- Thomas S., Fell D. A. (1998) The role of multiple enzyme activation in metabolic flux control. Adv. Enzyme Regul. 38, 65–85 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

