Inhibition of a novel specific neuroglial integrin signaling pathway increases STAT3-mediated CNTF expression
- PMID: 23693126
- PMCID: PMC3691611
- DOI: 10.1186/1478-811X-11-35
Inhibition of a novel specific neuroglial integrin signaling pathway increases STAT3-mediated CNTF expression
Abstract
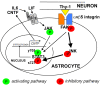
Background: Ciliary neurotrophic factor (CNTF) expression is repressed in astrocytes by neuronal contact in the CNS and is rapidly induced by injury. Here, we defined an inhibitory integrin signaling pathway.
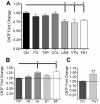
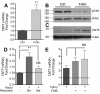
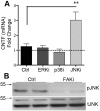
Results: The integrin substrates laminin, fibronectin and vitronectin, but not collagen, thrombospondin or fibrinogen, reduced CNTF expression in C6 astroglioma cells. Antibodies against αv and β5, but not α6 or β1, integrin induced CNTF. Together, the ligand and antibody specificity suggests that CNTF is repressed by αvβ5 integrin. Antibodies against Thy1, an abundant neuronal surface protein whose function is unclear, induced CNTF in neuron-astrocyte co-cultures indicating that it is a neuroglial CNTF repressor. Inhibition of the integrin signaling molecule Focal Adhesion Kinase (FAK) or the downstream c-Jun N-terminal kinase (JNK), but not extracellular regulated kinase (ERK) or p38 MAPK, greatly induced CNTF mRNA and protein expression within 4 hours. This selective inhibitory pathway phosphorylated STAT3 on its inhibitory ser-727 residue interfering with activity of the pro-transcription Tyr-705 residue. STAT3 can activate CNTF transcription because it bound to its promoter and FAK antagonist-induced CNTF was reduced by blocking STAT3. Microinjection of FAK inhibitor directly into the brain or spinal cord in adult mice rapidly induced CNTF mRNA and protein expression. Importantly, systemic treatment with FAK inhibitors over 3 days induced CNTF in the subventricular zone and increased neurogenesis.
Conclusions: Neuron-astroglia contact mediated by integrins serves as a sensor to enable rapid neurotrophic responses and provides a new pharmacological avenue to exploit the neuroprotective properties of endogenous CNTF.
Figures

 : CNTF initiation site;
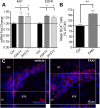
: CNTF initiation site;  ; < > denote DNA regions excluded from this panel for presentation purposes. E) IL-6 treatment of C6 cells for 15 minutes robustly increased phosphorylation of STAT3 at the Tyr-705 residue (y705) with modest increases after CNTF and LIF as shown by western blot. Ser-727 phosphorylation (s727) or total STAT3 (tSTAT3) was not affected. Similar results were seen at 4 hours. The blot is representative of 4 independent experiments. F) IL-6 induced only an ~10% increase in CNTF mRNA expression in C6 cells after 4 hours and did not augment FAKi-induced CNTF expression (n = 3-4 each, p < 0.05). G) FAK inhibition reduced phosphorylation of STAT3 (y705) in C6 cells most notably under IL-6 treated conditions. Antibodies against total STAT3 were used as internal controls for western blots. Results were repeatable in independent experiments.
; < > denote DNA regions excluded from this panel for presentation purposes. E) IL-6 treatment of C6 cells for 15 minutes robustly increased phosphorylation of STAT3 at the Tyr-705 residue (y705) with modest increases after CNTF and LIF as shown by western blot. Ser-727 phosphorylation (s727) or total STAT3 (tSTAT3) was not affected. Similar results were seen at 4 hours. The blot is representative of 4 independent experiments. F) IL-6 induced only an ~10% increase in CNTF mRNA expression in C6 cells after 4 hours and did not augment FAKi-induced CNTF expression (n = 3-4 each, p < 0.05). G) FAK inhibition reduced phosphorylation of STAT3 (y705) in C6 cells most notably under IL-6 treated conditions. Antibodies against total STAT3 were used as internal controls for western blots. Results were repeatable in independent experiments.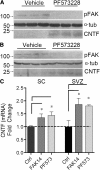
Similar articles
-
Inhibition of astrocyte FAK-JNK signaling promotes subventricular zone neurogenesis through CNTF.Glia. 2018 Nov;66(11):2456-2469. doi: 10.1002/glia.23498. Glia. 2018. PMID: 30500112 Free PMC article.
-
Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia.J Neurosci. 2000 Jun 1;20(11):4081-90. doi: 10.1523/JNEUROSCI.20-11-04081.2000. J Neurosci. 2000. PMID: 10818143 Free PMC article.
-
Integrin-FAK signaling rapidly and potently promotes mitochondrial function through STAT3.Cell Commun Signal. 2016 Dec 15;14(1):32. doi: 10.1186/s12964-016-0157-7. Cell Commun Signal. 2016. PMID: 27978828 Free PMC article.
-
Loss of neuron-astroglial interaction rapidly induces protective CNTF expression after stroke in mice.J Neurosci. 2012 Jul 4;32(27):9277-87. doi: 10.1523/JNEUROSCI.1746-12.2012. J Neurosci. 2012. PMID: 22764235 Free PMC article.
-
The Roles of Ciliary Neurotrophic Factor - from Neuronutrition to Energy Metabolism.Protein Pept Lett. 2022;29(10):815-828. doi: 10.2174/0929866529666220905105800. Protein Pept Lett. 2022. PMID: 36065930 Review.
Cited by
-
Vitronectin from brain pericytes promotes adult forebrain neurogenesis by stimulating CNTF.Exp Neurol. 2019 Feb;312:20-32. doi: 10.1016/j.expneurol.2018.11.002. Epub 2018 Nov 6. Exp Neurol. 2019. PMID: 30408465 Free PMC article.
-
MiR-9-5p Down-Regulates PiT2, but not PiT1 in Human Embryonic Kidney 293 Cells.J Mol Neurosci. 2017 May;62(1):28-33. doi: 10.1007/s12031-017-0906-0. Epub 2017 Mar 16. J Mol Neurosci. 2017. PMID: 28303467
-
LZK-dependent stimulation of astrocyte reactivity promotes corticospinal axon sprouting.Front Cell Neurosci. 2022 Sep 15;16:969261. doi: 10.3389/fncel.2022.969261. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36187291 Free PMC article.
-
Protective Functions of Reactive Astrocytes Following Central Nervous System Insult.Front Immunol. 2020 Sep 30;11:573256. doi: 10.3389/fimmu.2020.573256. eCollection 2020. Front Immunol. 2020. PMID: 33117368 Free PMC article. Review.
-
Astrocytic role of Thy-1 induced inhibition of axonal sprouting.Neural Regen Res. 2021 Jun;16(6):1192-1193. doi: 10.4103/1673-5374.300429. Neural Regen Res. 2021. PMID: 33269771 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

