A single HIV-1 cluster and a skewed immune homeostasis drive the early spread of HIV among resting CD4+ cell subsets within one month post-infection
- PMID: 23691172
- PMCID: PMC3653877
- DOI: 10.1371/journal.pone.0064219
A single HIV-1 cluster and a skewed immune homeostasis drive the early spread of HIV among resting CD4+ cell subsets within one month post-infection
Abstract
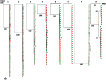
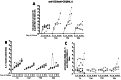
Optimizing therapeutic strategies for an HIV cure requires better understanding the characteristics of early HIV-1 spread among resting CD4+ cells within the first month of primary HIV-1 infection (PHI). We studied the immune distribution, diversity, and inducibility of total HIV-DNA among the following cell subsets: monocytes, peripheral blood activated and resting CD4 T cells, long-lived (naive [TN] and central-memory [TCM]) and short-lived (transitional-memory [TTM] and effector-memory cells [TEM]) resting CD4+T cells from 12 acutely-infected individuals recruited at a median 36 days from infection. Cells were sorted for total HIV-DNA quantification, phylogenetic analysis and inducibility, all studied in relation to activation status and cell signaling. One month post-infection, a single CCR5-restricted viral cluster was massively distributed in all resting CD4+ subsets from 88% subjects, while one subject showed a slight diversity. High levels of total HIV-DNA were measured among TN (median 3.4 log copies/million cells), although 10-fold less (p = 0.0005) than in equally infected TCM (4.5), TTM (4.7) and TEM (4.6) cells. CD3-CD4+ monocytes harbored a low viral burden (median 2.3 log copies/million cells), unlike equally infected resting and activated CD4+ T cells (4.5 log copies/million cells). The skewed repartition of resting CD4 subsets influenced their contribution to the pool of resting infected CD4+T cells, two thirds of which consisted of short-lived TTM and TEM subsets, whereas long-lived TN and TCM subsets contributed the balance. Each resting CD4 subset produced HIV in vitro after stimulation with anti-CD3/anti-CD28+IL-2 with kinetics and magnitude varying according to subset differentiation, while IL-7 preferentially induced virus production from long-lived resting TN cells. In conclusion, within a month of infection, a clonal HIV-1 cluster is massively distributed among resting CD4 T-cell subsets with a flexible inducibility, suggesting that subset activation and skewed immune homeostasis determine the conditions of viral dissemination and early establishment of the HIV reservoir.
Conflict of interest statement
Figures


Similar articles
-
Atlas of the HIV-1 Reservoir in Peripheral CD4 T Cells of Individuals on Successful Antiretroviral Therapy.mBio. 2021 Dec 21;12(6):e0307821. doi: 10.1128/mBio.03078-21. Epub 2021 Nov 30. mBio. 2021. PMID: 34844430 Free PMC article.
-
Establishment and Reversal of HIV-1 Latency in Naive and Central Memory CD4+ T Cells In Vitro.J Virol. 2016 Aug 26;90(18):8059-73. doi: 10.1128/JVI.00553-16. Print 2016 Sep 15. J Virol. 2016. PMID: 27356901 Free PMC article.
-
Initiation of Antiretroviral Therapy Restores CD4+ T Memory Stem Cell Homeostasis in Simian Immunodeficiency Virus-Infected Macaques.J Virol. 2016 Jul 11;90(15):6699-6708. doi: 10.1128/JVI.00492-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27170752 Free PMC article.
-
Comprehensive Mass Cytometry Analysis of Cell Cycle, Activation, and Coinhibitory Receptors Expression in CD4 T Cells from Healthy and HIV-Infected Individuals.Cytometry B Clin Cytom. 2017 Jan;92(1):21-32. doi: 10.1002/cyto.b.21502. Cytometry B Clin Cytom. 2017. PMID: 27997758 Review.
-
[Deep lung--cellular reaction to HIV].Rev Port Pneumol. 2007 Mar-Apr;13(2):175-212. Rev Port Pneumol. 2007. PMID: 17492233 Review. Portuguese.
Cited by
-
More than the Infinite Monkey Theorem: NHP Models in the Development of a Pediatric HIV Cure.Curr HIV/AIDS Rep. 2024 Feb;21(1):11-29. doi: 10.1007/s11904-023-00686-6. Epub 2024 Jan 16. Curr HIV/AIDS Rep. 2024. PMID: 38227162 Free PMC article. Review.
-
Targeting noncoding RNAs to reactivate or eliminate latent HIV reservoirs.Curr Opin HIV AIDS. 2024 Mar 1;19(2):47-55. doi: 10.1097/COH.0000000000000838. Epub 2023 Dec 20. Curr Opin HIV AIDS. 2024. PMID: 38169367 Review.
-
Single-cell RNA sequencing reveals common and unique gene expression profiles in primary CD4+ T cells latently infected with HIV under different conditions.Front Cell Infect Microbiol. 2023 Dec 12;13:1286168. doi: 10.3389/fcimb.2023.1286168. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38156317 Free PMC article.
-
Development of an HIV reporter virus that identifies latently infected CD4+ T cells.Cell Rep Methods. 2022 Jun 13;2(6):100238. doi: 10.1016/j.crmeth.2022.100238. eCollection 2022 Jun 20. Cell Rep Methods. 2022. PMID: 35784650 Free PMC article.
-
Ex Vivo Differentiation of Resting CD4+ T Lymphocytes Enhances Detection of Replication Competent HIV-1 in Viral Outgrowth Assays.Methods Mol Biol. 2022;2407:315-331. doi: 10.1007/978-1-0716-1871-4_21. Methods Mol Biol. 2022. PMID: 34985673
References
-
- Blankson JN, Persaud D, Siliciano RF (2002) The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med 53: 557–593. - PubMed
-
- Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, et al. (2013) Comparative Analysis of Measures of Viral Reservoirs in HIV-1 Eradication Studies. PLoS Pathogens 9(2): e1003174 doi:10.1371/journal.ppat.1003174. - DOI - PMC - PubMed
-
- Goujard C, Bonarek M, Meyer L, Bonnet F, Chaix ML, et al. (2006) CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin Infect Dis 42: 709–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

