Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections
- PMID: 23680933
- PMCID: PMC3779529
- DOI: 10.1016/j.nano.2013.05.003
Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections
Abstract
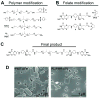
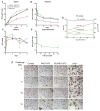
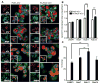
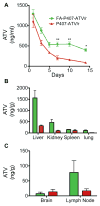
Macrophages serve as vehicles for the carriage and delivery of polymer-coated nanoformulated antiretroviral therapy (nanoART). Although superior to native drug, high drug concentrations are required for viral inhibition. Herein, folate-modified ritonavir-boosted atazanavir (ATV/r)-encased polymers facilitated macrophage receptor targeting for optimizing drug dosing. Folate coating of nanoART ATV/r significantly enhanced cell uptake, retention and antiretroviral activities without altering cell viability. Enhanced retentions of folate-coated nanoART within recycling endosomes provided a stable subcellular drug depot. Importantly, up to a five-fold enhanced plasma and tissue drug levels followed folate-coated formulation injection in mice. Folate polymer encased ATV/r improves nanoART pharmacokinetics bringing the technology one step closer to human use.
From the clinical editor: This team of authors describes a novel method for macrophage folate receptor-targeted antiretroviral therapy. Atazanvir entry, retention, and antiretroviral activities were superior using the presented method, and so was its biodistribution, enabling a more efficient way to address human immunodeficiency virus infections, with a hoped for clinical application in the near future.
Keywords: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; ATV; ATV/r; BSA; CHO; Chinese hamster ovary; FA; FOLR; Folate; Human immunodeficiency virus; LAMP; MDM; MTT; Macrophages; NanoART; PBS; PDI; PFA; Poloxamer 407; RME; RT; RTV; Targeted drug delivery; atazanavir; bovine serum albumin; folate receptor; folic acid; lysosomal associated membrane protein; monocyte-derived macrophages; nanoART; nanoformulated antiretroviral therapeutics; paraformaldehyde; phosphate buffered saline; polydispersity index; receptor-mediated endocytosis; reverse transcriptase; ritonavir; ritonavir-boosted atazanavir.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
Endosomal trafficking of nanoformulated antiretroviral therapy facilitates drug particle carriage and HIV clearance.J Virol. 2014 Sep 1;88(17):9504-13. doi: 10.1128/JVI.01557-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920821 Free PMC article.
-
Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy.Antimicrob Agents Chemother. 2013 Jul;57(7):3110-20. doi: 10.1128/AAC.00267-13. Epub 2013 Apr 22. Antimicrob Agents Chemother. 2013. PMID: 23612193 Free PMC article.
-
Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells.Int J Nanomedicine. 2012;7:2373-88. doi: 10.2147/IJN.S29454. Epub 2012 May 8. Int J Nanomedicine. 2012. PMID: 22661891 Free PMC article.
-
Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir.Clin Pharmacokinet. 2005;44(10):1035-50. doi: 10.2165/00003088-200544100-00003. Clin Pharmacokinet. 2005. PMID: 16176117 Review.
-
A systematic review of the use of atazanavir in women infected with HIV-1.Antivir Ther. 2014;19(3):293-307. doi: 10.3851/IMP2742. Epub 2014 Jan 31. Antivir Ther. 2014. PMID: 24480797 Review.
Cited by
-
Optimizing the preparation and stability of decorated antiretroviral drug nanocrystals.Nanomedicine (Lond). 2018 Apr;13(8):871-885. doi: 10.2217/nnm-2017-0381. Epub 2018 Mar 19. Nanomedicine (Lond). 2018. PMID: 29553879 Free PMC article.
-
The Promise of Long-Acting Antiretroviral Therapies: From Need to Manufacture.Trends Microbiol. 2019 Jul;27(7):593-606. doi: 10.1016/j.tim.2019.02.009. Epub 2019 Apr 10. Trends Microbiol. 2019. PMID: 30981593 Free PMC article. Review.
-
Targeting of Hepatic Macrophages by Therapeutic Nanoparticles.Front Immunol. 2020 Mar 4;11:218. doi: 10.3389/fimmu.2020.00218. eCollection 2020. Front Immunol. 2020. PMID: 32194546 Free PMC article. Review.
-
Endosomal trafficking of nanoformulated antiretroviral therapy facilitates drug particle carriage and HIV clearance.J Virol. 2014 Sep 1;88(17):9504-13. doi: 10.1128/JVI.01557-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920821 Free PMC article.
-
Gallium nanoparticles facilitate phagosome maturation and inhibit growth of virulent Mycobacterium tuberculosis in macrophages.PLoS One. 2017 May 18;12(5):e0177987. doi: 10.1371/journal.pone.0177987. eCollection 2017. PLoS One. 2017. PMID: 28542623 Free PMC article.
References
-
- Este JA, Cihlar T. Current status and challenges of antiretroviral research and therapy. Antiviral Res. 2010;85:25–33. - PubMed
-
- Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns. 2002;46:93–108. - PubMed
-
- Chesney M. Adherence to HAART regimens. AIDS Patient Care STDS. 2003;17:169–177. - PubMed
-
- Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376:49–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- P01 NS31492/NS/NINDS NIH HHS/United States
- P01 DA026146/DA/NIDA NIH HHS/United States
- 2R01 NS034239/NS/NINDS NIH HHS/United States
- P01 MH64570/MH/NIMH NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- 9P20GM103480/GM/NIGMS NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- R01 NS036127/NS/NINDS NIH HHS/United States
- R13 NS083315/NS/NINDS NIH HHS/United States
- P20 GM103480/GM/NIGMS NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- P01 NS43985/NS/NINDS NIH HHS/United States
- R01 NS36126/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

