VAMP4 is required to maintain the ribbon structure of the Golgi apparatus
- PMID: 23677696
- PMCID: PMC3695666
- DOI: 10.1007/s11010-013-1652-4
VAMP4 is required to maintain the ribbon structure of the Golgi apparatus
Erratum in
- Mol Cell Biochem. 2013 Aug;380(1-2):301. Shakakura, Yasunori [corrected to Sakakura, Yasunori]
Abstract
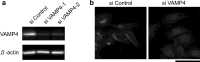
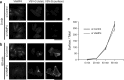
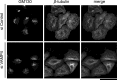
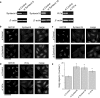
The Golgi apparatus forms a twisted ribbon-like network in the juxtanuclear region of vertebrate cells. Vesicle-associated membrane protein 4 (VAMP4), a v-SNARE protein expressed exclusively in the vertebrate trans-Golgi network (TGN), plays a role in retrograde trafficking from the early endosome to the TGN, although its precise function within the Golgi apparatus remains unclear. To determine whether VAMP4 plays a functional role in maintaining the structure of the Golgi apparatus, we depleted VAMP4 gene expression using RNA interference technology. Depletion of VAMP4 from HeLa cells led to fragmentation of the Golgi ribbon. These fragments were not uniformly distributed throughout the cytoplasm, but remained in the juxtanuclear area. Electron microscopy and immunohistochemistry showed that in the absence of VAMP4, the length of the Golgi stack was shortened, but Golgi stacking was normal. Anterograde trafficking was not impaired in VAMP4-depleted cells, which contained intact microtubule arrays. Depletion of the cognate SNARE partners of VAMP4, syntaxin 6, syntaxin 16, and Vti1a also disrupted the Golgi ribbon structure. Our findings suggested that the maintenance of Golgi ribbon structure requires normal retrograde trafficking from the early endosome to the TGN, which is likely to be mediated by the formation of VAMP4-containing SNARE complexes.
Figures



Similar articles
-
The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport.J Cell Biol. 2011 Aug 8;194(3):459-72. doi: 10.1083/jcb.201102045. Epub 2011 Aug 1. J Cell Biol. 2011. PMID: 21807881 Free PMC article.
-
The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure.Traffic. 2007 Jun;8(6):758-73. doi: 10.1111/j.1600-0854.2007.00563.x. Epub 2007 May 4. Traffic. 2007. PMID: 17488291
-
The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network.Mol Biol Cell. 2007 Dec;18(12):4979-91. doi: 10.1091/mbc.e07-06-0622. Epub 2007 Oct 3. Mol Biol Cell. 2007. PMID: 17914056 Free PMC article.
-
Coiled-coil interactions are required for post-Golgi R-SNARE trafficking.EMBO Rep. 2009 Aug;10(8):851-6. doi: 10.1038/embor.2009.96. Epub 2009 Jun 26. EMBO Rep. 2009. PMID: 19557002 Free PMC article. Review.
-
Syntaxin 16's Newly Deciphered Roles in Autophagy.Cells. 2019 Dec 17;8(12):1655. doi: 10.3390/cells8121655. Cells. 2019. PMID: 31861136 Free PMC article. Review.
Cited by
-
Endosomal MR1 Trafficking Plays a Key Role in Presentation of Mycobacterium tuberculosis Ligands to MAIT Cells.PLoS Pathog. 2016 Mar 31;12(3):e1005524. doi: 10.1371/journal.ppat.1005524. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 27031111 Free PMC article.
-
BAIAP3, a C2 domain-containing Munc13 protein, controls the fate of dense-core vesicles in neuroendocrine cells.J Cell Biol. 2017 Jul 3;216(7):2151-2166. doi: 10.1083/jcb.201702099. Epub 2017 Jun 16. J Cell Biol. 2017. PMID: 28626000 Free PMC article.
-
Systemic analysis of the prognostic significance and interaction network of miR-26b-3p in cholangiocarcinoma.Appl Biochem Biotechnol. 2024 Jul;196(7):4166-4187. doi: 10.1007/s12010-023-04753-x. Epub 2023 Nov 2. Appl Biochem Biotechnol. 2024. PMID: 37914963
-
Synaptic Vesicle Recycling and the Endolysosomal System: A Reappraisal of Form and Function.Front Synaptic Neurosci. 2022 Feb 25;14:826098. doi: 10.3389/fnsyn.2022.826098. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35280702 Free PMC article. Review.
-
Sec22b determines Weibel-Palade body length by controlling anterograde ER-Golgi transport.Haematologica. 2021 Apr 1;106(4):1138-1147. doi: 10.3324/haematol.2019.242727. Haematologica. 2021. PMID: 32336681 Free PMC article.
References
-
- Mironov A, Pavelka M. The Golgi apparatus: state of the art 110 years after Camillo Golgi’s discovery. In: De Matteis MA, Mironov AA, Beznoussenko GV, editors. The Golgi ribbon and the function of the golgins. New York: Springer; 2008. pp. 223–246.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

