Annexin A1: A new immunohistological marker of cholangiocarcinoma
- PMID: 23674846
- PMCID: PMC3646135
- DOI: 10.3748/wjg.v19.i16.2456
Annexin A1: A new immunohistological marker of cholangiocarcinoma
Abstract
Aim: To evaluate a new immunohistological marker, annexin A1 (ANXA1), in cholangiocarcinoma (CCA) and hepatocellular carcinoma (HCC).
Methods: Expression of ANXA1 protein was investigated in liver tissues from patients with CCA and HCC by immunohistochemistry. Its expression on differences stages of tumor development was investigated in hamster CCA tissues induced by Opisthorchis viverrini and N-nitrosodimethylamine. Moreover, mRNA expression of ANXA1 was assessed in CCA cell lines by quantitative real-time polymerase chain reaction and silencing of ANXA1 gene expression using small interfering RNA.
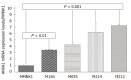
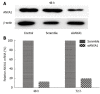
Results: In human CCA tissue arrays, immunohistochemical analysis revealed that the positive expression of ANXA1 was 94.1% (64/68 cases) consisting of a high expression (66.2%, 45/68 cases) and a low expression (33.8%, 23/68 cases). However, expression of ANXA1 protein was negative in all histologic patterns for HCC (46/46 cases) and healthy individuals (6/6 cases). In hamster with opisthorchiasis-associated CCA, the expression of ANXA1 was observed in the cytoplasm of inflammatory cells, bile duct epithelia and tumor cells. Grading scores of ANXA1 expression were significantly increased with tumor progression. In addition, mRNA expression of ANXA1 significantly increased in all of the various CCA cell lines tested compared to an immortalized human cholangiocyte cell line (MMNK1). Suppressing the ANXA1 gene significantly reduced the matrix metalloproteinase (MMP) 2 and MMP9, and transforming growth factor-β genes, but increased nuclear factor-κB gene expression.
Conclusion: ANXA1 is highly expressed in CCA, but low in HCC, suggesting it may serve as a new immunohistochemical marker of CCA. ANXA1 may play a role in opisthorchiasis-associated cholangiocarcinogenesis.
Keywords: Annexin A1; Biomarker; Cholangiocarcinoma; Hepatocellular carcinoma; Opisthorchis viverrini.
Figures





Similar articles
-
Overexpression of PDGFA and its receptor during carcinogenesis of Opisthorchis viverrini-associated cholangiocarcinoma.Parasitol Int. 2012 Mar;61(1):145-50. doi: 10.1016/j.parint.2011.07.008. Epub 2011 Jul 14. Parasitol Int. 2012. PMID: 21777692
-
Involvement of c-Ski oncoprotein in carcinogenesis of cholangiocacinoma induced by Opisthorchis viverrini and N-nitrosodimethylamine.Pathol Oncol Res. 2011 Jun;17(2):219-27. doi: 10.1007/s12253-010-9300-8. Epub 2010 Sep 18. Pathol Oncol Res. 2011. PMID: 20853076
-
Expression of oxysterol binding protein isoforms in opisthorchiasis-associated cholangiocarcinoma: a potential molecular marker for tumor metastasis.Parasitol Int. 2012 Mar;61(1):136-9. doi: 10.1016/j.parint.2011.07.003. Epub 2011 Jul 8. Parasitol Int. 2012. PMID: 21763455
-
[Control of Opisthorchis viverrini infection for cholangiocarcinoma prevention].Bull Soc Pathol Exot. 2017 Feb;110(1):61-67. doi: 10.1007/s13149-017-0544-8. Epub 2017 Jan 19. Bull Soc Pathol Exot. 2017. PMID: 28105582 Review. French.
-
Risk biomarkers for assessment and chemoprevention of liver fluke-associated cholangiocarcinoma.J Hepatobiliary Pancreat Sci. 2014 May;21(5):309-15. doi: 10.1002/jhbp.63. Epub 2014 Jan 10. J Hepatobiliary Pancreat Sci. 2014. PMID: 24408859 Review.
Cited by
-
Expression levels and prognostic values of annexins in liver cancer.Oncol Lett. 2019 Dec;18(6):6657-6669. doi: 10.3892/ol.2019.11025. Epub 2019 Oct 30. Oncol Lett. 2019. PMID: 31807177 Free PMC article.
-
ANXA1 is identified as a key gene associated with high risk and T cell infiltration in primary sclerosing cholangitis.Hum Genomics. 2023 Sep 21;17(1):86. doi: 10.1186/s40246-023-00534-z. Hum Genomics. 2023. PMID: 37735492 Free PMC article.
-
Identification of functional modules by integration of multiple data sources using a Bayesian network classifier.Circ Cardiovasc Genet. 2014 Apr;7(2):206-17. doi: 10.1161/CIRCGENETICS.113.000087. Circ Cardiovasc Genet. 2014. PMID: 24736851 Free PMC article.
-
Immunoproteomics approach revealed elevated autoantibody levels against ANXA1 in early stage gallbladder carcinoma.BMC Cancer. 2020 Dec 1;20(1):1175. doi: 10.1186/s12885-020-07676-6. BMC Cancer. 2020. PMID: 33261560 Free PMC article.
-
Annexin Expression in Cholangiocarcinoma, and Metastatic Pancreatic Ductal Adenocarcinoma "Is it be Helpful for Differential Diagnosis of These Tumors in the Liver?".Iran J Pathol. 2021 Fall;16(4):433-438. doi: 10.30699/IJP.20201.138489.2512. Epub 2021 Jul 6. Iran J Pathol. 2021. PMID: 34567193 Free PMC article.
References
-
- Kamsa-ard S, Wiangnon S, Suwanrungruang K, Promthet S, Khuntikeo N, Kamsa-ard S, Mahaweerawat S. Trends in liver cancer incidence between 1985 and 2009, Khon Kaen, Thailand: cholangiocarcinoma. Asian Pac J Cancer Prev. 2011;12:2209–2213. - PubMed
-
- Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. - PubMed
-
- Sriamporn S, Pisani P, Pipitgool V, Suwanrungruang K, Kamsa-ard S, Parkin DM. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004;9:588–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

