Mycoplasma pneumoniae CARDS toxin is internalized via clathrin-mediated endocytosis
- PMID: 23667510
- PMCID: PMC3647021
- DOI: 10.1371/journal.pone.0062706
Mycoplasma pneumoniae CARDS toxin is internalized via clathrin-mediated endocytosis
Abstract
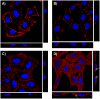
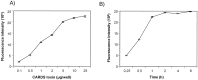
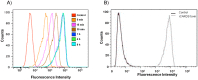
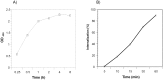
Bacterial toxins possess specific mechanisms of binding and uptake by mammalian cells. Mycoplasma pneumoniae CARDS (Community Acquired Respiratory Distress Syndrome) toxin is a 68 kDa protein, which demonstrates high binding affinity to human surfactant protein-A and exhibits specific biological activities including mono-ADP ribosylation and vacuolization. These properties lead to inflammatory processes in the airway and a range of cytopathologies including ciliostasis, loss of tissue integrity and injury, and cell death. However, the process by which CARDS toxin enters target cells is unknown. In this study, we show that CARDS toxin binds to mammalian cell surfaces and is internalized rapidly in a dose and time-dependent manner using a clathrin-mediated pathway, as indicated by inhibition of toxin internalization by monodansylcadaverine but not by methyl-β-cyclodextrin or filipin. Furthermore, the internalization of CARDS toxin was markedly inhibited in clathrin-depleted cells.
Conflict of interest statement
Figures
Similar articles
-
Annexin A2 mediates Mycoplasma pneumoniae community-acquired respiratory distress syndrome toxin binding to eukaryotic cells.mBio. 2014 Aug 19;5(4):e01497-14. doi: 10.1128/mBio.01497-14. mBio. 2014. PMID: 25139904 Free PMC article.
-
Exploring the pathogenicity of Mycoplasma pneumoniae: Focus on community-acquired respiratory distress syndrome toxins.Microb Pathog. 2024 Oct;195:106865. doi: 10.1016/j.micpath.2024.106865. Epub 2024 Aug 15. Microb Pathog. 2024. PMID: 39153578 Review.
-
Mycoplasma pneumoniae Community-Acquired Respiratory Distress Syndrome Toxin Uses a Novel KELED Sequence for Retrograde Transport and Subsequent Cytotoxicity.mBio. 2018 Jan 23;9(1):e01663-17. doi: 10.1128/mBio.01663-17. mBio. 2018. PMID: 29362229 Free PMC article.
-
Mycoplasma pneumoniae Community Acquired Respiratory Distress Syndrome toxin expression reveals growth phase and infection-dependent regulation.Mol Microbiol. 2010 Jun 1;76(5):1127-41. doi: 10.1111/j.1365-2958.2010.07092.x. Epub 2010 Feb 28. Mol Microbiol. 2010. PMID: 20199607 Free PMC article.
-
Pathogenesis of Mycoplasma pneumoniae: An update.Indian J Med Microbiol. 2016 Jan-Mar;34(1):7-16. doi: 10.4103/0255-0857.174112. Indian J Med Microbiol. 2016. PMID: 26776112 Review.
Cited by
-
Community-Acquired Respiratory Distress Syndrome Toxin: Unique Exotoxin for M. pneumoniae.Front Microbiol. 2021 Nov 19;12:766591. doi: 10.3389/fmicb.2021.766591. eCollection 2021. Front Microbiol. 2021. PMID: 34867898 Free PMC article. Review.
-
Potential Molecular Targets for Narrow-Spectrum Agents to Combat Mycoplasma pneumoniae Infection and Disease.Front Microbiol. 2016 Feb 25;7:205. doi: 10.3389/fmicb.2016.00205. eCollection 2016. Front Microbiol. 2016. PMID: 26941728 Free PMC article. Review.
-
ADP-ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS toxin regulates inflammasome activity.mBio. 2014 Dec 23;5(6):e02186-14. doi: 10.1128/mBio.02186-14. mBio. 2014. PMID: 25538194 Free PMC article.
-
Mycoplasma pneumoniae CARDS toxin exploits host cell endosomal acidic pH and vacuolar ATPase proton pump to execute its biological activities.Sci Rep. 2021 Jun 2;11(1):11571. doi: 10.1038/s41598-021-90948-3. Sci Rep. 2021. PMID: 34078958 Free PMC article.
-
Global research trends of Mycoplasma pneumoniae pneumonia in children: a bibliometric analysis.Front Pediatr. 2023 Nov 24;11:1306234. doi: 10.3389/fped.2023.1306234. eCollection 2023. Front Pediatr. 2023. PMID: 38078315 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

