Angiopoietin-like 2 promotes atherogenesis in mice
- PMID: 23666461
- PMCID: PMC3698785
- DOI: 10.1161/JAHA.113.000201
Angiopoietin-like 2 promotes atherogenesis in mice
Abstract
Background: Angiopoietin like-2 (angptl2), a proinflammatory protein, is overexpressed in endothelial cells (ECs) from patients with coronary artery disease (CAD). Whether angptl2 contributes to atherogenesis is unknown. We tested the hypothesis that angptl2 promotes inflammation and leukocyte adhesion onto ECs, thereby accelerating atherogenesis in preatherosclerotic dyslipidemic mice.
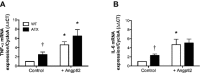
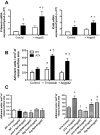
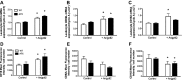
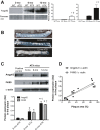
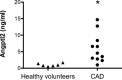
Methods and results: In ECs freshly isolated from the aorta, basal expression of TNF-α and IL-6 mRNA was higher in 3-month-old severely dyslipidemic mice (LDLr(-/-); hApoB100(+/+) [ATX]) than in control healthy wild-type (WT) mice (P<0.05) and was increased in both groups by exogenous angptl2 (100 nmol/L). Angptl2 stimulated the adhesion of leukocytes ex vivo on the native aortic endothelium of ATX, but not WT mice, in association with higher expression of ICAM-1 and P-selectin in ECs (P<0.05). Antibodies against these endothelial adhesion molecules prevented leukocyte adhesion. Intravenous administration of angptl2 for 1 month in preatherosclerotic 3-month-old ATX mice increased (P<0.05) total cholesterol and LDL-cholesterol levels, strongly induced (P<0.05) the expression of endothelial proinflammatory cytokines and adhesion molecules while accelerating atherosclerotic lesion formation by 10-fold (P<0.05). Plasma and aortic tissue levels of angptl2 increased (P<0.05) with age and were higher in 6- and 12-month-old ATX mice than in age-matched WT mice. Angptl2 accumulated to high levels in the atherosclerotic lesions (P<0.05). Finally, angptl2 was greatly expressed (P<0.05) in ECs cultured from CAD patients, and circulating angptl2 levels were 6-fold higher in CAD patients compared with age-matched healthy volunteers.
Conclusions: Angptl2 contributes to the pathogenesis of atherosclerosis.
Keywords: CAD; adhesion molecules; aging; freshly isolated mouse endothelial cells; inflammation; mouse model of atherosclerosis.
Figures

Similar articles
-
Knockdown of angiopoietin-like 2 induces clearance of vascular endothelial senescent cells by apoptosis, promotes endothelial repair and slows atherogenesis in mice.Aging (Albany NY). 2019 Jun 11;11(11):3832-3850. doi: 10.18632/aging.102020. Aging (Albany NY). 2019. PMID: 31186381 Free PMC article.
-
High Circulating Levels of ANGPTL2: Beyond a Clinical Marker of Systemic Inflammation.Oxid Med Cell Longev. 2017;2017:1096385. doi: 10.1155/2017/1096385. Epub 2017 Aug 24. Oxid Med Cell Longev. 2017. PMID: 29138671 Free PMC article. Review.
-
Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):790-800. doi: 10.1161/ATVBAHA.113.303116. Epub 2014 Feb 13. Arterioscler Thromb Vasc Biol. 2014. PMID: 24526691
-
Angiopoietin-like protein 2 mediates endotoxin-induced acute inflammation in the eye.Lab Invest. 2012 Nov;92(11):1553-63. doi: 10.1038/labinvest.2012.111. Epub 2012 Aug 6. Lab Invest. 2012. PMID: 22868908
-
Leukocyte adhesion molecules in atherogenesis.Clin Chim Acta. 1999 Aug;286(1-2):207-18. doi: 10.1016/s0009-8981(99)00102-3. Clin Chim Acta. 1999. PMID: 10511293 Review.
Cited by
-
Knockdown of angiopoietin-like 2 induces clearance of vascular endothelial senescent cells by apoptosis, promotes endothelial repair and slows atherogenesis in mice.Aging (Albany NY). 2019 Jun 11;11(11):3832-3850. doi: 10.18632/aging.102020. Aging (Albany NY). 2019. PMID: 31186381 Free PMC article.
-
High Circulating Levels of ANGPTL2: Beyond a Clinical Marker of Systemic Inflammation.Oxid Med Cell Longev. 2017;2017:1096385. doi: 10.1155/2017/1096385. Epub 2017 Aug 24. Oxid Med Cell Longev. 2017. PMID: 29138671 Free PMC article. Review.
-
Angiopoietin-like Protein 2 Is a Multistep Regulator of Inflammatory Neovascularization in a Murine Model of Age-related Macular Degeneration.J Biol Chem. 2016 Apr 1;291(14):7373-85. doi: 10.1074/jbc.M115.710186. Epub 2016 Feb 2. J Biol Chem. 2016. PMID: 26839315 Free PMC article.
-
Angiopoietin-like 2 has auxo-action in atherosclerosis by promoting atherosclerotic calcification.Int J Clin Exp Pathol. 2017 Aug 1;10(8):9084-9091. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966781 Free PMC article.
-
ANGPTL2 knockdown induces autophagy to relieve alveolar macrophage pyroptosis by reducing LILRB2-mediated inhibition of TREM2.J Cell Mol Med. 2024 May;28(10):e18280. doi: 10.1111/jcmm.18280. J Cell Mol Med. 2024. PMID: 38758159 Free PMC article.
References
-
- Hato T, Tabata M, Oike Y. The role of angiopoietin‐like proteins in angiogenesis and metabolism. Trends Cardiovasc Med. 2008; 18:6-14 - PubMed
-
- Morisada T, Kubota Y, Urano T, Suda T, Oike Y. Angiopoietins and angiopoietin‐like proteins in angiogenesis. Endothelium. 2006; 13:71-79 - PubMed
-
- Ando Y, Shimizugawa T, Takeshita S, Ono M, Shimamura M, Koishi R, Furukawa H. A decreased expression of angiopoietin‐like 3 is protective against atherosclerosis in ApoE‐deficient mice. J Lipid Res. 2003; 44:1216-1223 - PubMed
-
- Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hedner T, Corella D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton‐Cheh C, Salomaa V, Peltonen L, Groop L, Altshuler DM, Orho‐Melander M. Six new loci associated with blood low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008; 40:189-197 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

