EphrinA5-EphA7 complex induces apoptotic cell death via TNFR1
- PMID: 23657875
- PMCID: PMC3887865
- DOI: 10.1007/s10059-013-0072-3
EphrinA5-EphA7 complex induces apoptotic cell death via TNFR1
Abstract
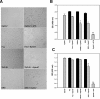
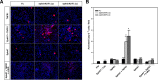
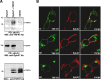
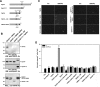
A previous study showed that the EphA7 receptor regulates apoptotic cell death during early brain development. In this study, we provide evidence that the EphA7 receptor interacts with death receptors such as tumor necrosis factor receptor 1 (TNFR1) to decrease cell viability. We showed that ephrinA5 stimulates EphA7 to activate the TNFR1-mediated apoptotic signaling pathway. In addition, a pull-down assay using biotinylated ephrinA5-Fc revealed that ephrinA5-EphA7 complexes recruit TNFR1 to form a multi-protein complex. Immunocytochemical staining analysis showed that EphA7 was co-localized with TNFR1 on the cell surface when cells were incubated with ephrinA5 at low temperatures. Finally, both the internalization motif and death domain of TNFR1 was important for interacting with an intracytoplasmic region of EphA7; this interaction was essential for inducing the apoptotic signaling cascade. This result suggests that a distinct multi-protein complex comprising ephrinA5, EphA7, and TNFR1 may constitute a platform for inducing caspase-dependent apoptotic cell death.
Figures
Similar articles
-
EphA receptors form a complex with caspase-8 to induce apoptotic cell death.Mol Cells. 2015 Apr;38(4):349-55. doi: 10.14348/molcells.2015.2279. Mol Cells. 2015. PMID: 25855521 Free PMC article.
-
Ligand-dependent EphA7 signaling inhibits prostate tumor growth and progression.Cell Death Dis. 2017 Oct 12;8(10):e3122. doi: 10.1038/cddis.2017.507. Cell Death Dis. 2017. PMID: 29022918 Free PMC article.
-
Dlx5 and Msx2 regulate mouse anterior neural tube closure through ephrinA5-EphA7.Dev Growth Differ. 2013 Apr;55(3):341-9. doi: 10.1111/dgd.12044. Epub 2013 Feb 21. Dev Growth Differ. 2013. PMID: 23425387
-
The role of EphA7 in different tumors.Clin Transl Oncol. 2022 Jul;24(7):1274-1289. doi: 10.1007/s12094-022-02783-1. Epub 2022 Feb 2. Clin Transl Oncol. 2022. PMID: 35112312 Review.
-
Poly-ubiquitination in TNFR1-mediated necroptosis.Cell Mol Life Sci. 2016 Jun;73(11-12):2165-76. doi: 10.1007/s00018-016-2191-4. Epub 2016 Apr 11. Cell Mol Life Sci. 2016. PMID: 27066894 Free PMC article. Review.
Cited by
-
EphA receptors form a complex with caspase-8 to induce apoptotic cell death.Mol Cells. 2015 Apr;38(4):349-55. doi: 10.14348/molcells.2015.2279. Mol Cells. 2015. PMID: 25855521 Free PMC article.
-
Eph receptors and ephrins in cancer progression.Nat Rev Cancer. 2024 Jan;24(1):5-27. doi: 10.1038/s41568-023-00634-x. Epub 2023 Nov 23. Nat Rev Cancer. 2024. PMID: 37996538 Free PMC article. Review.
-
Ligand-dependent EphA7 signaling inhibits prostate tumor growth and progression.Cell Death Dis. 2017 Oct 12;8(10):e3122. doi: 10.1038/cddis.2017.507. Cell Death Dis. 2017. PMID: 29022918 Free PMC article.
-
Unbiased identification of novel transcription factors in striatal compartmentation and striosome maturation.Elife. 2021 Oct 5;10:e65979. doi: 10.7554/eLife.65979. Elife. 2021. PMID: 34609283 Free PMC article.
-
Erythropoietin-producing hepatocellular A7 triggering ovulation indicates a potential beneficial role for polycystic ovary syndrome.EBioMedicine. 2018 Oct;36:539-552. doi: 10.1016/j.ebiom.2018.09.046. Epub 2018 Oct 3. EBioMedicine. 2018. PMID: 30292674 Free PMC article.
References
-
- Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, Coffman K, Jackson D, Bruckheimer E, Muraoka-Cook RS, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. - PMC - PubMed
-
- Ciossek T, Millauer B, Ullrich A. Identification of alternatively spliced mRNAs encoding variants of MDK1, a novel receptor tyrosine kinase expressed in the murine nervous system. Oncogene. 1995;10:97–108. - PubMed
-
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci. 2000;23:454–458. - PubMed
-
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. - PubMed
-
- Gu C, Shim S, Shin J, Kim J, Park J, Han K, Park S. The EphA8 receptor induces sustained MAP kinase activation to promote neurite outgrowth in neuronal cells. Oncogene. 2005;24:4243–4256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

