Ever closer to a prophylactic vaccine for HCV
- PMID: 23651228
- PMCID: PMC3741017
- DOI: 10.1517/14712598.2013.791277
Ever closer to a prophylactic vaccine for HCV
Abstract
Introduction: With 3 - 4 million new infections occurring annually, hepatitis C virus (HCV) is a major global health problem. There is increasing evidence to suggest that HCV will be highly amenable to a vaccine approach, and despite advances in treatment, a vaccine remains the most cost-effective and realistic means to significantly reduce the worldwide mortality and morbidity associated with persistent HCV infection.
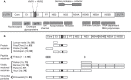
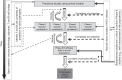
Areas covered: In this review we discuss immune responses to HCV during natural infection, and describe how they may inform vaccine design. We introduce the current candidate vaccines for HCV and compare how these fare against the expected requirements of an effective prophylactic HCV vaccine in relation to the breadth, functionality, magnitude and phenotype of the vaccine-induced immune response.
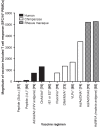
Expert opinion: Although the correlates of immune protection against HCV are not completely defined, we now have vaccine technologies capable of inducing HCV-specific adaptive immune responses to an order of magnitude that are associated with protection during natural infection. The challenge next is to i) establish well-characterised cohorts of people at risk of HCV infection for vaccine efficacy testing and ii) to better understand the correlates of protection in natural history studies. If these can be achieved, a vaccine against HCV appears a realistic goal.
Figures
Similar articles
-
Methods to Evaluate Novel Hepatitis C Virus Vaccines.Methods Mol Biol. 2016;1403:221-44. doi: 10.1007/978-1-4939-3387-7_11. Methods Mol Biol. 2016. PMID: 27076133
-
Approaches, Progress, and Challenges to Hepatitis C Vaccine Development.Gastroenterology. 2019 Jan;156(2):418-430. doi: 10.1053/j.gastro.2018.08.060. Epub 2018 Sep 27. Gastroenterology. 2019. PMID: 30268785 Free PMC article. Review.
-
Challenges and Promise of a Hepatitis C Virus Vaccine.Cold Spring Harb Perspect Med. 2020 Feb 3;10(2):a036947. doi: 10.1101/cshperspect.a036947. Cold Spring Harb Perspect Med. 2020. PMID: 31548228 Free PMC article. Review.
-
Advances in hepatitis C virus vaccines, Part one: Advances in basic knowledge for hepatitis C virus vaccine design.Expert Opin Ther Pat. 2011 Dec;21(12):1811-30. doi: 10.1517/13543776.2011.630662. Epub 2011 Oct 25. Expert Opin Ther Pat. 2011. PMID: 22022980 Review.
-
Hepatitis C vaccine: supply and demand.Lancet Infect Dis. 2008 Jun;8(6):379-86. doi: 10.1016/S1473-3099(08)70126-9. Lancet Infect Dis. 2008. PMID: 18501853
Cited by
-
Efficacy and Safety of Ribavirin with Sofosbuvir Plus Ledipasvir in Patients with Genotype 1 Hepatitis C: A Meta-Analysis.Dig Dis Sci. 2016 Nov;61(11):3108-3117. doi: 10.1007/s10620-016-4291-2. Epub 2016 Sep 12. Dig Dis Sci. 2016. PMID: 27619394 Free PMC article. Review.
-
Global distribution and prevalence of hepatitis C virus genotypes.Hepatology. 2015 Jan;61(1):77-87. doi: 10.1002/hep.27259. Epub 2014 Jul 28. Hepatology. 2015. PMID: 25069599 Free PMC article.
-
Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination.Proc Natl Acad Sci U S A. 2015 Mar 10;112(10):3050-5. doi: 10.1073/pnas.1500475112. Epub 2015 Feb 23. Proc Natl Acad Sci U S A. 2015. PMID: 25713354 Free PMC article.
-
Viral genotype correlates with distinct liver gene transcription signatures in chronic hepatitis C virus infection.Liver Int. 2015 Oct;35(10):2256-64. doi: 10.1111/liv.12830. Epub 2015 Apr 7. Liver Int. 2015. PMID: 25800823 Free PMC article.
-
Combined adenovirus vector and hepatitis C virus envelope protein prime-boost regimen elicits T cell and neutralizing antibody immune responses.J Virol. 2014 May;88(10):5502-10. doi: 10.1128/JVI.03574-13. Epub 2014 Mar 5. J Virol. 2014. PMID: 24599994 Free PMC article.
References
-
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. - PubMed
-
- WHO WHO fact sheet: HCV vaccines number 164. http://www.who.int/mediacentre/factsheets/fs164/en/ 2012 Available from: [Last] [accessed 07 February 2013]
-
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–15. - PubMed
-
- Dahari H, Feinstone SM, Major ME. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology. 2010;139:965–74. - PMC - PubMed
-
This article presents a meta-analysis of candidate prophylactic vaccines for HCV tested in the Chimpanzee challenge model.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
