Cardiac resynchronization therapy improves altered Na channel gating in canine model of dyssynchronous heart failure
- PMID: 23650309
- PMCID: PMC4114055
- DOI: 10.1161/CIRCEP.113.000400
Cardiac resynchronization therapy improves altered Na channel gating in canine model of dyssynchronous heart failure
Abstract
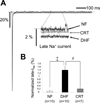
Background: Slowed Na⁺ current (INa) decay and enhanced late INa (INa-L) prolong the action potential duration (APD) and contribute to early afterdepolarizations. Cardiac resynchronization therapy (CRT) shortens APD compared with dyssynchronous heart failure (DHF); however, the role of altered Na⁺ channel gating in CRT remains unexplored.
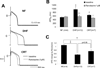
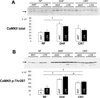
Methods and results: Adult dogs underwent left-bundle branch ablation and right atrial pacing (200 beats/min) for 6 weeks (DHF) or 3 weeks followed by 3 weeks of biventricular pacing at the same rate (CRT). INa and INa-L were measured in left ventricular myocytes from nonfailing, DHF, and CRT dogs. DHF shifted voltage-dependence of INa availability by -3 mV compared with nonfailing, enhanced intermediate inactivation, and slowed recovery from inactivation. CRT reversed the DHF-induced voltage shift of availability, partially reversed enhanced intermediate inactivation but did not affect DHF-induced slowed recovery. DHF markedly increased INa-L compared with nonfailing. CRT dramatically reduced DHF-induced enhanced INa-L, abbreviated the APD, and suppressed early afterdepolarizations. CRT was associated with a global reduction in phosphorylated Ca²⁺/Calmodulin protein kinase II, which has distinct effects on inactivation of cardiac Na⁺ channels. In a canine AP model, alterations of INa-L are sufficient to reproduce the effects on APD observed in DHF and CRT myocytes.
Conclusions: CRT improves DHF-induced alterations of Na⁺ channel function, especially suppression of INa-L, thus, abbreviating the APD and reducing the frequency of early afterdepolarizations. Changes in the levels of phosphorylated Ca²⁺/Calmodulin protein kinase II suggest a molecular pathway for regulation of INa by biventricular pacing of the failing heart.
Keywords: Na+ channels; arrhythmias; cardiac resynchronization therapy; electrophysiology; heart failure.
Conflict of interest statement
Figures





Similar articles
-
Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy.Circulation. 2009 Mar 10;119(9):1220-30. doi: 10.1161/CIRCULATIONAHA.108.794834. Epub 2009 Feb 23. Circulation. 2009. PMID: 19237662 Free PMC article.
-
Electrical remodeling in dyssynchrony and resynchronization.J Cardiovasc Transl Res. 2012 Apr;5(2):170-9. doi: 10.1007/s12265-012-9348-9. Epub 2012 Jan 21. J Cardiovasc Transl Res. 2012. PMID: 22271011 Review.
-
Electrical remodeling in the failing heart.Curr Opin Cardiol. 2010 Jan;25(1):29-36. doi: 10.1097/HCO.0b013e328333d3d6. Curr Opin Cardiol. 2010. PMID: 19907317 Free PMC article. Review.
-
Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization.Circulation. 2008 Mar 18;117(11):1369-77. doi: 10.1161/CIRCULATIONAHA.107.706291. Epub 2008 Mar 3. Circulation. 2008. PMID: 18316490
-
Cardiac Resynchronization Therapy Reduces Subcellular Heterogeneity of Ryanodine Receptors, T-Tubules, and Ca2+ Sparks Produced by Dyssynchronous Heart Failure.Circ Heart Fail. 2015 Nov;8(6):1105-14. doi: 10.1161/CIRCHEARTFAILURE.115.002352. Epub 2015 Aug 20. Circ Heart Fail. 2015. PMID: 26294422 Free PMC article.
Cited by
-
Myocyte repolarization modulates myocardial function in aging dogs.Am J Physiol Heart Circ Physiol. 2016 Apr 1;310(7):H873-90. doi: 10.1152/ajpheart.00682.2015. Epub 2016 Jan 22. Am J Physiol Heart Circ Physiol. 2016. PMID: 26801307 Free PMC article.
-
Rationale and design of the IRON-CRT trial: effect of intravenous ferric carboxymaltose on reverse remodelling following cardiac resynchronization therapy.ESC Heart Fail. 2019 Dec;6(6):1208-1215. doi: 10.1002/ehf2.12503. Epub 2019 Sep 28. ESC Heart Fail. 2019. PMID: 31562751 Free PMC article. Clinical Trial.
-
Cardiac resynchronization therapy reduces expression of inflammation-promoting genes related to interleukin-1β in heart failure.Cardiovasc Res. 2020 Jun 1;116(7):1311-1322. doi: 10.1093/cvr/cvz232. Cardiovasc Res. 2020. PMID: 31612215 Free PMC article.
-
Cellular and Molecular Aspects of Dyssynchrony and Resynchronization.Card Electrophysiol Clin. 2015 Dec;7(4):585-97. doi: 10.1016/j.ccep.2015.08.011. Card Electrophysiol Clin. 2015. PMID: 26596804 Free PMC article. Review.
-
New drug discovery of cardiac anti-arrhythmic drugs: insights in animal models.Sci Rep. 2023 Sep 29;13(1):16420. doi: 10.1038/s41598-023-41942-4. Sci Rep. 2023. PMID: 37775650 Free PMC article.
References
-
- Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. - PubMed
-
- Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. - PubMed
-
- Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. - PubMed
-
- Kääb S, Nuss HB, Chiamvimonvat N, O’Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–273. - PubMed
-
- Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

