LKB1 controls human bronchial epithelial morphogenesis through p114RhoGEF-dependent RhoA activation
- PMID: 23648482
- PMCID: PMC3700127
- DOI: 10.1128/MCB.00154-13
LKB1 controls human bronchial epithelial morphogenesis through p114RhoGEF-dependent RhoA activation
Abstract
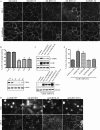
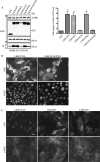
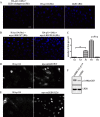
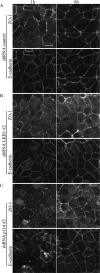
LKB1 is a Ser/Thr kinase that plays an important role in controlling both energy metabolism and cell polarity in metazoan organisms. LKB1 is also a tumor suppressor, and homozygous, inactivating mutations are found in a wide range of human cancers. In lung cancer, inactivating mutations are found in 10 to 50% of cases, but the consequences of functional loss in this context are poorly understood. We report here that LKB1 is required for the maturation of apical junctions in the human bronchial epithelial cell line 16HBE14o- (16HBE). This activity is dependent on an interaction with the Rho guanine nucleotide exchange factor p114RhoGEF but is independent of LKB1 kinase activity. Together, LKB1 and p114RhoGEF control RhoA activity in these cells to promote apical junction assembly.
Figures



Similar articles
-
Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis.Nat Cell Biol. 2011 Feb;13(2):159-66. doi: 10.1038/ncb2156. Epub 2011 Jan 23. Nat Cell Biol. 2011. PMID: 21258369 Free PMC article.
-
G Protein betagamma subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production.Circ Res. 2003 Oct 31;93(9):848-56. doi: 10.1161/01.RES.0000097607.14733.0C. Epub 2003 Sep 25. Circ Res. 2003. PMID: 14512443
-
Involvement of p114-RhoGEF and Lfc in Wnt-3a- and dishevelled-induced RhoA activation and neurite retraction in N1E-115 mouse neuroblastoma cells.Mol Biol Cell. 2010 Oct 15;21(20):3590-600. doi: 10.1091/mbc.E10-02-0095. Epub 2010 Sep 1. Mol Biol Cell. 2010. PMID: 20810787 Free PMC article.
-
The many faces of the guanine-nucleotide exchange factor trio.Cell Adh Migr. 2012 Nov-Dec;6(6):482-7. doi: 10.4161/cam.21418. Epub 2012 Oct 17. Cell Adh Migr. 2012. PMID: 23076143 Free PMC article. Review.
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
-
LKB1 kinase-dependent and -independent defects disrupt polarity and adhesion signaling to drive collagen remodeling during invasion.Mol Biol Cell. 2016 Apr 1;27(7):1069-84. doi: 10.1091/mbc.E15-08-0569. Epub 2016 Feb 10. Mol Biol Cell. 2016. PMID: 26864623 Free PMC article.
-
Genetic Predisposition to Breast and Ovarian Cancers: How Many and Which Genes to Test?Int J Mol Sci. 2020 Feb 8;21(3):1128. doi: 10.3390/ijms21031128. Int J Mol Sci. 2020. PMID: 32046255 Free PMC article. Review.
-
Celiac Disease: Role of the Epithelial Barrier.Cell Mol Gastroenterol Hepatol. 2017 Jan 14;3(2):150-162. doi: 10.1016/j.jcmgh.2016.12.006. eCollection 2017 Mar. Cell Mol Gastroenterol Hepatol. 2017. PMID: 28275682 Free PMC article. Review.
-
Association between a Single Nucleotide Polymorphism in the 3'-UTR of ARHGEF18 and the Risk of Nonidiopathic Pulmonary Arterial Hypertension in Chinese Population.Dis Markers. 2018 Oct 14;2018:2461845. doi: 10.1155/2018/2461845. eCollection 2018. Dis Markers. 2018. PMID: 30405854 Free PMC article.
-
Expression of novel "LOCGEF" isoforms of ARHGEF18 in eosinophils.J Leukoc Biol. 2018 Jul;104(1):135-145. doi: 10.1002/JLB.2MA1017-418RR. Epub 2018 Mar 30. J Leukoc Biol. 2018. PMID: 29601110 Free PMC article.
References
-
- Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W, Trimbath JD, Giardiello FM, Gruber SB, Offerhaus GJ, de Rooij FW, Wilson JH, Hansmann A, Moslein G, Royer-Pokora B, Vogel T, Phillips RK, Spigelman AD, Houlston RS. 2006. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin. Cancer Res. 12:3209–3215 - PubMed
-
- Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, Shimamura T, Miller DS, Sharpless NE, Bardeesy N, Kwiatkowski DJ, Schorge JO, Wong KK, Castrillon DH. 2009. Somatic LKB1 mutations promote cervical cancer progression. PLoS One 4:e5137.10.1371/journal.pone.0005137 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
