APP/PS1 mice overexpressing SREBP-2 exhibit combined Aβ accumulation and tau pathology underlying Alzheimer's disease
- PMID: 23648430
- PMCID: PMC3736868
- DOI: 10.1093/hmg/ddt201
APP/PS1 mice overexpressing SREBP-2 exhibit combined Aβ accumulation and tau pathology underlying Alzheimer's disease
Abstract
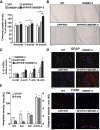
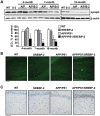
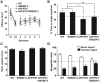
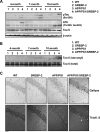
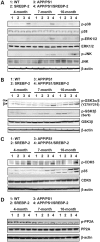
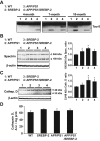
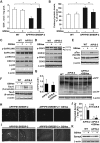
Current evidence indicates that excess brain cholesterol regulates amyloid-β (Aβ) deposition, which in turn can regulate cholesterol homeostasis. Moreover, Aβ neurotoxicity is potentiated, in part, by mitochondrial glutathione (mGSH) depletion. To better understand the relationship between alterations in cholesterol homeostasis and Alzheimer's disease (AD), we generated a triple transgenic mice featuring sterol regulatory element-binding protein-2 (SREBP-2) overexpression in combination with APPswe/PS1ΔE9 mutations (APP/PS1) to examine key biochemical and functional characteristics of AD. Unlike APP/PS1 mice, APP/PS1/SREBP-2 mice exhibited early mitochondrial cholesterol loading and mGSH depletion. Moreover, β-secretase activation and Aβ accumulation, correlating with oxidative damage and neuroinflammation, were accelerated in APP/PS1/SREBP-2 mice compared with APP/PS1 mice. Triple transgenic mice displayed increased synaptotoxicity reflected by loss of synaptophysin and neuronal death, resulting in early object-recognition memory impairment associated with deficits in spatial memory. Interestingly, tau pathology was present in APP/PS1/SREBP-2 mice, manifested by increased tau hyperphosphorylation and cleavage, activation of tau kinases and neurofibrillary tangle (NFT) formation without expression of mutated tau. Importantly, in vivo treatment with the cell permeable GSH ethyl ester, which restored mGSH levels in APP/PS1/SREBP-2 mice, partially prevented the activation of tau kinases, reduced abnormal tau aggregation and Aβ deposition, resulting in attenuated synaptic degeneration. Taken together, these results show that cholesterol-mediated mGSH depletion is a key event in AD progression, accelerating the onset of key neuropathological hallmarks of the disease. Thus, therapeutic approaches to recover mGSH may represent a relevant strategy in the treatment of AD.
Figures

Similar articles
-
Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity.J Neurosci. 2009 May 20;29(20):6394-405. doi: 10.1523/JNEUROSCI.4909-08.2009. J Neurosci. 2009. PMID: 19458211 Free PMC article.
-
ABCA7 Deficiency Accelerates Amyloid-β Generation and Alzheimer's Neuronal Pathology.J Neurosci. 2016 Mar 30;36(13):3848-59. doi: 10.1523/JNEUROSCI.3757-15.2016. J Neurosci. 2016. PMID: 27030769 Free PMC article.
-
Modeling Alzheimer's disease in mouse without mutant protein overexpression: cooperative and independent effects of Aβ and tau.PLoS One. 2013 Nov 20;8(11):e80706. doi: 10.1371/journal.pone.0080706. eCollection 2013. PLoS One. 2013. PMID: 24278307 Free PMC article.
-
Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review.
-
Roles of glycogen synthase kinase 3 in Alzheimer's disease.Curr Alzheimer Res. 2012 Sep;9(7):864-79. doi: 10.2174/156720512802455386. Curr Alzheimer Res. 2012. PMID: 22272620 Review.
Cited by
-
A Novel Tetramethylpyrazine Derivative Prophylactically Protects against Glutamate-Induced Excitotoxicity in Primary Neurons through the Blockage of N-Methyl-D-aspartate Receptor.Front Pharmacol. 2018 Feb 12;9:73. doi: 10.3389/fphar.2018.00073. eCollection 2018. Front Pharmacol. 2018. PMID: 29483871 Free PMC article.
-
Increasing membrane cholesterol of neurons in culture recapitulates Alzheimer's disease early phenotypes.Mol Neurodegener. 2014 Dec 18;9:60. doi: 10.1186/1750-1326-9-60. Mol Neurodegener. 2014. PMID: 25524049 Free PMC article.
-
Role of LDL cholesterol and endolysosomes in amyloidogenesis and Alzheimer's disease.J Neurol Neurophysiol. 2014 Oct 1;5(5):236. doi: 10.4172/2155-9562.1000236. J Neurol Neurophysiol. 2014. PMID: 26413387 Free PMC article.
-
The Protective Role of Selenium on Scopolamine-Induced Memory Impairment, Oxidative Stress, and Apoptosis in Aged Rats: The Involvement of TRPM2 and TRPV1 Channels.Mol Neurobiol. 2017 May;54(4):2852-2868. doi: 10.1007/s12035-016-9835-0. Epub 2016 Mar 28. Mol Neurobiol. 2017. PMID: 27021021
-
Mitochondrial Glutathione: Recent Insights and Role in Disease.Antioxidants (Basel). 2020 Sep 24;9(10):909. doi: 10.3390/antiox9100909. Antioxidants (Basel). 2020. PMID: 32987701 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

