Mammalian target of rapamycin (mTOR) pathways in neurological diseases
- PMID: 23644232
- PMCID: PMC3880546
- DOI: 10.4103/2319-4170.110365
Mammalian target of rapamycin (mTOR) pathways in neurological diseases
Abstract
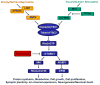
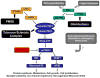
The mammalian target of rapamycin (mTOR) pathway is an essential cellular signaling pathway involved in a number of important physiological functions, including cell growth, proliferation, metabolism, protein synthesis, and autophagy. Dysregulation of the mTOR pathway has been implicated in the pathophysiology of a number of neurological diseases. Hyperactivation of the mTOR pathway, leading to increased cell growth and proliferation, has been most convincingly shown to stimulate tumor growth in the brain and other organs in the genetic disorder, tuberous sclerosis complex (TSC). In addition, mTOR may also play a role in promoting epileptogenesis or maintaining seizures in TSC, as well as in acquired epilepsies following brain injury. Finally, the mTOR pathway may also be involved in the pathogenesis of cognitive dysfunction and other neurological deficits in developmental disorders and neurodegenerative diseases. mTOR inhibitors, such as rapamycin and its analogs, may represent novel, rational therapies for a variety of neurological disorders.
Figures
Similar articles
-
Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies.Epilepsia. 2010 Jan;51(1):27-36. doi: 10.1111/j.1528-1167.2009.02341.x. Epub 2009 Oct 8. Epilepsia. 2010. PMID: 19817806 Free PMC article. Review.
-
mTOR Inhibitors in Children: Current Indications and Future Directions in Neurology.Curr Neurol Neurosci Rep. 2016 Dec;16(12):102. doi: 10.1007/s11910-016-0708-8. Curr Neurol Neurosci Rep. 2016. PMID: 27815691 Review.
-
Mechanistic target of rapamycin (mTOR) in tuberous sclerosis complex-associated epilepsy.Pediatr Neurol. 2015 Mar;52(3):281-9. doi: 10.1016/j.pediatrneurol.2014.10.028. Epub 2014 Nov 20. Pediatr Neurol. 2015. PMID: 25591831 Review.
-
The role of mTOR inhibitors in preventing epileptogenesis in patients with TSC: Current evidence and future perspectives.Epilepsy Behav. 2019 Feb;91:94-98. doi: 10.1016/j.yebeh.2018.05.039. Epub 2018 Jun 22. Epilepsy Behav. 2019. PMID: 29941212 Review.
-
Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex.Neuro Oncol. 2015 Dec;17(12):1550-9. doi: 10.1093/neuonc/nov152. Epub 2015 Aug 19. Neuro Oncol. 2015. PMID: 26289591 Free PMC article. Review.
Cited by
-
Protective Effects of AGE and Its Components on Neuroinflammation and Neurodegeneration.Neuromolecular Med. 2016 Sep;18(3):474-82. doi: 10.1007/s12017-016-8410-1. Epub 2016 Jun 4. Neuromolecular Med. 2016. PMID: 27263111
-
Developing Novel Experimental Models of m-TORopathic Epilepsy and Related Neuropathologies: Translational Insights from Zebrafish.Int J Mol Sci. 2023 Jan 12;24(2):1530. doi: 10.3390/ijms24021530. Int J Mol Sci. 2023. PMID: 36675042 Free PMC article. Review.
-
[Chinese expert consensus on surgical treatment of tuberous sclerosis complex-related epilepsy].Zhongguo Dang Dai Er Ke Za Zhi. 2019 Aug;21(8):735-742. doi: 10.7499/j.issn.1008-8830.2019.08.001. Zhongguo Dang Dai Er Ke Za Zhi. 2019. PMID: 31416495 Free PMC article. Chinese. No abstract available.
-
Sin1-mediated mTOR signaling in cell growth, metabolism and immune response.Natl Sci Rev. 2019 Nov;6(6):1149-1162. doi: 10.1093/nsr/nwz171. Epub 2019 Nov 19. Natl Sci Rev. 2019. PMID: 34691993 Free PMC article.
-
Proteomic quantification and site-mapping of S-nitrosylated proteins using isobaric iodoTMT reagents.J Proteome Res. 2014 Jul 3;13(7):3200-11. doi: 10.1021/pr401179v. Epub 2014 Jun 13. J Proteome Res. 2014. PMID: 24926564 Free PMC article.
References
-
- Dazert E, Hall MN. mTOR signaling in disease. Current Opin Cell Biol. 2011;23:744–755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
