The use of the Cre/loxP system to study oxidative stress in tissue-specific manganese superoxide dismutase knockout models
- PMID: 23641945
- PMCID: PMC3942694
- DOI: 10.1089/ars.2013.5293
The use of the Cre/loxP system to study oxidative stress in tissue-specific manganese superoxide dismutase knockout models
Abstract
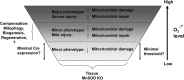
Significance: Respiring mitochondria are a significant site for reactions involving reactive oxygen and nitrogen species that contribute to irreversible cellular, structural, and functional damage leading to multiple pathological conditions. Manganese superoxide dismutase (MnSOD) is a critical component of the antioxidant system tasked with protecting the oxidant-sensitive mitochondrial compartment from oxidative stress. Since global knockout of MnSOD results in significant cardiac and neuronal damage leading to early postnatal lethality, this approach has limited use for studying the mechanisms of oxidant stress and the development of disease in specific tissues lacking MnSOD. To circumvent this problem, a number of investigators have employed the Cre/loxP system to precisely knockout MnSOD in individual tissues.
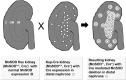
Recent advances: Multiple tissue and organ-specific Cre-expressing mice have been generated, which greatly enhance the specificity of MnSOD knockout in tissues and organ systems that were once difficult, if not impossible to study.
Critical issues: Evaluating the contribution of MnSOD deficiency to oxidant-mediated mitochondrial damage requires careful consideration of the promoter system used for creating the tissue-specific knockout animal, in addition to the collection and interpretation of multiple indices of oxidative stress and damage.
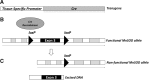
Future directions: Expanded use of well-characterized tissue-specific promoter elements and inducible systems to drive the Cre/loxP recombinational events will lead to a spectrum of MnSOD tissue knockout models, and a clearer understanding of the role of MnSOD in preventing mitochondrial dysfunction in human disease.
Figures


Similar articles
-
Bladder function in mice with inducible smooth muscle-specific deletion of the manganese superoxide dismutase gene.Am J Physiol Cell Physiol. 2015 Aug 1;309(3):C169-78. doi: 10.1152/ajpcell.00046.2015. Epub 2015 May 6. Am J Physiol Cell Physiol. 2015. PMID: 25948732 Free PMC article.
-
Model mice for tissue-specific deletion of the manganese superoxide dismutase (MnSOD) gene.Biochem Biophys Res Commun. 2002 Aug 23;296(3):729-36. doi: 10.1016/s0006-291x(02)00933-6. Biochem Biophys Res Commun. 2002. PMID: 12176043
-
Absence of manganese superoxide dismutase delays p53-induced tumor formation.Redox Biol. 2014 Jan 13;2:220-3. doi: 10.1016/j.redox.2014.01.001. eCollection 2014. Redox Biol. 2014. PMID: 24494196 Free PMC article.
-
Managing odds in stem cells: insights into the role of mitochondrial antioxidant enzyme MnSOD.Free Radic Res. 2016;50(5):570-84. doi: 10.3109/10715762.2016.1155708. Free Radic Res. 2016. PMID: 26899340 Review.
-
Invited review: manganese superoxide dismutase in disease.Free Radic Res. 2001 Apr;34(4):325-36. doi: 10.1080/10715760100300281. Free Radic Res. 2001. PMID: 11328670 Review.
Cited by
-
The Type and Source of Reactive Oxygen Species Influences the Outcome of Oxidative Stress in Cultured Cells.Cells. 2021 Apr 30;10(5):1075. doi: 10.3390/cells10051075. Cells. 2021. PMID: 33946545 Free PMC article.
-
Superoxide Dismutase Administration: A Review of Proposed Human Uses.Molecules. 2021 Mar 25;26(7):1844. doi: 10.3390/molecules26071844. Molecules. 2021. PMID: 33805942 Free PMC article.
-
Distinct Cell-specific Roles of NOX2 and MyD88 in Epileptogenesis.Front Cell Dev Biol. 2022 Jul 4;10:926776. doi: 10.3389/fcell.2022.926776. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35859905 Free PMC article. Review.
-
Highly efficient CRISPR/Cas9-mediated transgene knockin at the H11 locus in pigs.Sci Rep. 2015 Sep 18;5:14253. doi: 10.1038/srep14253. Sci Rep. 2015. PMID: 26381350 Free PMC article.
-
Bladder function in mice with inducible smooth muscle-specific deletion of the manganese superoxide dismutase gene.Am J Physiol Cell Physiol. 2015 Aug 1;309(3):C169-78. doi: 10.1152/ajpcell.00046.2015. Epub 2015 May 6. Am J Physiol Cell Physiol. 2015. PMID: 25948732 Free PMC article.
References
-
- Agnihotri N, Kaur S, Dilawari JB, Bhusnurmath SR, and Kaur U. Diminution in parietal cell number in experimental portal hypertensive gastropathy. Dig Dis Sci 42: 431–439, 1997 - PubMed
-
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med 10Suppl: S18–S25, 2004 - PubMed
-
- Birling MC, Gofflot F, and Warot X. Site-specific recombinases for manipulation of the mouse genome. Methods Mol Biol 561: 245–263, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

