Identification and analysis of multi-protein complexes in placenta
- PMID: 23638173
- PMCID: PMC3639281
- DOI: 10.1371/journal.pone.0062988
Identification and analysis of multi-protein complexes in placenta
Abstract
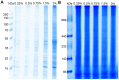
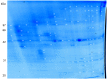
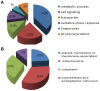
Placental malfunction induces pregnancy disorders which contribute to life-threatening complications for both the mother and the fetus. Identification and characterization of placental multi-protein complexes is an important step to integratedly understand the protein-protein interaction networks in placenta which determine placental function. In this study, blue native/sodium dodecyl sulfate polyacrylamide gel electrophoresis (BN/SDS-PAGE) and Liquid chromatography-tandem mass spectrometry (LC-MS/MS) were used to screen the multi-protein complexes in placenta. 733 unique proteins and 34 known and novel heterooligomeric multi-protein complexes including mitochondrial respiratory chain complexes, integrin complexes, proteasome complexes, histone complex, and heat shock protein complexes were identified. A novel protein complex, which involves clathrin and small conductance calcium-activated potassium (SK) channel protein 2, was identified and validated by antibody based gel shift assay, co-immunoprecipitation and immunofluorescence staining. These results suggest that BN/SDS-PAGE, when integrated with LC-MS/MS, is a very powerful and versatile tool for the investigation of placental protein complexes. This work paves the way for deeper functional characterization of the placental protein complexes associated with pregnancy disorders.
Conflict of interest statement
Figures

Similar articles
-
Analysis of expression and comparative profile of normal placental tissue proteins and those in preeclampsia patients using proteomic approaches.Anal Chim Acta. 2008 Nov 23;629(1-2):158-64. doi: 10.1016/j.aca.2008.09.015. Epub 2008 Sep 12. Anal Chim Acta. 2008. PMID: 18940332
-
Mono-dimensional blue native-PAGE and bi-dimensional blue native/urea-PAGE or/SDS-PAGE combined with nLC-ESI-LIT-MS/MS unveil membrane protein heteromeric and homomeric complexes in Streptococcus thermophilus.J Proteomics. 2013 Dec 6;94:240-61. doi: 10.1016/j.jprot.2013.09.007. Epub 2013 Sep 20. J Proteomics. 2013. PMID: 24061001
-
Expanded protein expression profile of human placenta using two-dimensional gel electrophoresis.Placenta. 2013 Feb;34(2):193-6. doi: 10.1016/j.placenta.2012.11.015. Epub 2012 Dec 20. Placenta. 2013. PMID: 23261269
-
[Application of "blue native" electrophoresis in the studies of mitochondrial respiratory chain complexes in physiology and pathology].Postepy Biochem. 2008;54(2):217-23. Postepy Biochem. 2008. PMID: 18807933 Review. Polish.
-
2D gel proteomics: an approach to study age-related differences in protein abundance or isoform complexity in biological samples.Methods Mol Biol. 2007;371:349-91. doi: 10.1007/978-1-59745-361-5_24. Methods Mol Biol. 2007. PMID: 17634592 Review.
Cited by
-
Extremely stable soluble high molecular mass multi-protein complex with DNase activity in human placental tissue.PLoS One. 2014 Nov 26;9(11):e111234. doi: 10.1371/journal.pone.0111234. eCollection 2014. PLoS One. 2014. PMID: 25426722 Free PMC article.
-
Blue Native PAGE-Antibody Shift in Conjunction with Mass Spectrometry to Reveal Protein Subcomplexes: Detection of a Cerebellar α1/α6-Subunits Containing γ-Aminobutyric Acid Type A Receptor Subtype.Int J Mol Sci. 2023 Apr 21;24(8):7632. doi: 10.3390/ijms24087632. Int J Mol Sci. 2023. PMID: 37108794 Free PMC article.
-
Protocol for Increasing the Sensitivity of MS-Based Protein Detection in Human Chorionic Villi.Curr Issues Mol Biol. 2022 May 9;44(5):2069-2088. doi: 10.3390/cimb44050140. Curr Issues Mol Biol. 2022. PMID: 35678669 Free PMC article.
-
Protease and DNase Activities of a Very Stable High-Molecular-Mass Multiprotein Complex from Sea Cucumber Eupentacta fraudatrix.Int J Mol Sci. 2022 Jun 15;23(12):6677. doi: 10.3390/ijms23126677. Int J Mol Sci. 2022. PMID: 35743119 Free PMC article.
-
Proteomic Approaches in the Study of Placenta of Pregnancy Complicated by Gestational Diabetes Mellitus.Biomedicines. 2022 Sep 14;10(9):2272. doi: 10.3390/biomedicines10092272. Biomedicines. 2022. PMID: 36140373 Free PMC article. Review.
References
-
- Garnica AD, Chan WY (1996) The role of the placenta in fetal nutrition and growth. J Am Coll Nutr 15: 206–222. - PubMed
-
- Mari G, Hanif F (2007) Intrauterine growth restriction: how to manage and when to deliver. Clin Obstet Gynecol 50: 497–509. - PubMed
-
- Johnstone ED, Sawicki G, Guilbert L, Winkler-Lowen B, Cadete VJ, et al. (2011) Differential proteomic analysis of highly purified placental cytotrophoblasts in pre-eclampsia demonstrates a state of increased oxidative stress and reduced cytotrophoblast antioxidant defense. Proteomics 11: 4077–4084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

