Factor H binds to the hypervariable region of many Streptococcus pyogenes M proteins but does not promote phagocytosis resistance or acute virulence
- PMID: 23637608
- PMCID: PMC3630203
- DOI: 10.1371/journal.ppat.1003323
Factor H binds to the hypervariable region of many Streptococcus pyogenes M proteins but does not promote phagocytosis resistance or acute virulence
Abstract
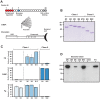
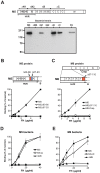
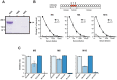
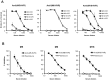
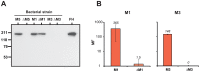
Many pathogens express a surface protein that binds the human complement regulator factor H (FH), as first described for Streptococcus pyogenes and the antiphagocytic M6 protein. It is commonly assumed that FH recruited to an M protein enhances virulence by protecting the bacteria against complement deposition and phagocytosis, but the role of FH-binding in S. pyogenes pathogenesis has remained unclear and controversial. Here, we studied seven purified M proteins for ability to bind FH and found that FH binds to the M5, M6 and M18 proteins but not the M1, M3, M4 and M22 proteins. Extensive immunochemical analysis indicated that FH binds solely to the hypervariable region (HVR) of an M protein, suggesting that selection has favored the ability of certain HVRs to bind FH. These FH-binding HVRs could be studied as isolated polypeptides that retain ability to bind FH, implying that an FH-binding HVR represents a distinct ligand-binding domain. The isolated HVRs specifically interacted with FH among all human serum proteins, interacted with the same region in FH and showed species specificity, but exhibited little or no antigenic cross-reactivity. Although these findings suggested that FH recruited to an M protein promotes virulence, studies in transgenic mice did not demonstrate a role for bound FH during acute infection. Moreover, phagocytosis tests indicated that ability to bind FH is neither sufficient nor necessary for S. pyogenes to resist killing in whole human blood. While these data shed new light on the HVR of M proteins, they suggest that FH-binding may affect S. pyogenes virulence by mechanisms not assessed in currently used model systems.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Binding of human C4BP to the hypervariable region of M protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes.Mol Microbiol. 2001 Oct;42(2):539-51. doi: 10.1046/j.1365-2958.2001.02664.x. Mol Microbiol. 2001. PMID: 11703674
-
Interaction between complement regulators and Streptococcus pyogenes: binding of C4b-binding protein and factor H/factor H-like protein 1 to M18 strains involves two different cell surface molecules.J Immunol. 2004 Dec 1;173(11):6899-904. doi: 10.4049/jimmunol.173.11.6899. J Immunol. 2004. PMID: 15557185
-
Complement factor H allotype 402H is associated with increased C3b opsonization and phagocytosis of Streptococcus pyogenes.Mol Microbiol. 2008 Nov;70(3):583-94. doi: 10.1111/j.1365-2958.2008.06347.x. Epub 2008 Jun 27. Mol Microbiol. 2008. PMID: 18627465
-
Variation, Indispensability, and Masking in the M protein.Trends Microbiol. 2018 Feb;26(2):132-144. doi: 10.1016/j.tim.2017.08.002. Epub 2017 Aug 31. Trends Microbiol. 2018. PMID: 28867148 Free PMC article. Review.
-
Host-pathogen interactions in Streptococcus pyogenes infections, with special reference to puerperal fever and a comment on vaccine development.Vaccine. 2004 Dec 6;22 Suppl 1:S9-S14. doi: 10.1016/j.vaccine.2004.08.010. Vaccine. 2004. PMID: 15576204 Review.
Cited by
-
A Role of Epithelial Cells and Virulence Factors in Biofilm Formation by Streptococcus pyogenes In Vitro.Infect Immun. 2020 Sep 18;88(10):e00133-20. doi: 10.1128/IAI.00133-20. Print 2020 Sep 18. Infect Immun. 2020. PMID: 32661124 Free PMC article.
-
Incremental Contributions of FbaA and Other Impetigo-Associated Surface Proteins to Fitness and Virulence of a Classical Group A Streptococcal Skin Strain.Infect Immun. 2017 Oct 18;85(11):e00374-17. doi: 10.1128/IAI.00374-17. Print 2017 Nov. Infect Immun. 2017. PMID: 28808160 Free PMC article.
-
Surface Proteins on Gram-Positive Bacteria.Microbiol Spectr. 2019 Jul;7(4):10.1128/microbiolspec.gpp3-0012-2018. doi: 10.1128/microbiolspec.GPP3-0012-2018. Microbiol Spectr. 2019. PMID: 31373270 Free PMC article. Review.
-
Responses of innate immune cells to group A Streptococcus.Front Cell Infect Microbiol. 2014 Oct 2;4:140. doi: 10.3389/fcimb.2014.00140. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25325020 Free PMC article. Review.
-
Factor H Family Proteins in Complement Evasion of Microorganisms.Front Immunol. 2017 May 18;8:571. doi: 10.3389/fimmu.2017.00571. eCollection 2017. Front Immunol. 2017. PMID: 28572805 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

