Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4
- PMID: 23637184
- PMCID: PMC3853377
- DOI: 10.1523/JNEUROSCI.1571-12.2013
Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4
Erratum in
- J Neurosci. 2013 Jun 19;33(25):10582. Lin, Ying-Xi [corrected to Lin, Yingxi]
Abstract
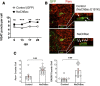
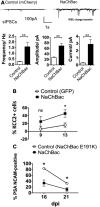
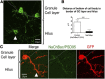
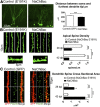
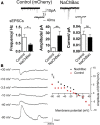
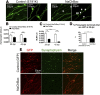
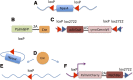
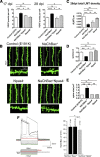
Electrical activity regulates the manner in which neurons mature and form connections to each other. However, it remains unclear whether increased single-cell activity is sufficient to alter the development of synaptic connectivity of that neuron or whether a global increase in circuit activity is necessary. To address this question, we genetically increased neuronal excitability of in vivo individual adult-born neurons in the mouse dentate gyrus via expression of a voltage-gated bacterial sodium channel. We observed that increasing the excitability of new neurons in an otherwise unperturbed circuit leads to changes in both their input and axonal synapses. Furthermore, the activity-dependent transcription factor Npas4 is necessary for the changes in the input synapses of these neurons, but it is not involved in changes to their axonal synapses. Our results reveal that an increase in cell-intrinsic activity during maturation is sufficient to alter the synaptic connectivity of a neuron with the hippocampal circuit and that Npas4 is required for activity-dependent changes in input synapses.
Figures

Similar articles
-
Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors.J Neurosci. 2012 May 30;32(22):7651-61. doi: 10.1523/JNEUROSCI.6246-11.2012. J Neurosci. 2012. PMID: 22649244 Free PMC article.
-
Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses.J Neurosci. 2005 Apr 20;25(16):4040-51. doi: 10.1523/JNEUROSCI.4115-04.2005. J Neurosci. 2005. PMID: 15843606 Free PMC article.
-
A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis.J Neurosci. 2011 Apr 6;31(14):5414-25. doi: 10.1523/JNEUROSCI.2456-10.2011. J Neurosci. 2011. PMID: 21471377 Free PMC article.
-
Synapse diversity and synaptome architecture in human genetic disorders.Hum Mol Genet. 2019 Nov 21;28(R2):R219-R225. doi: 10.1093/hmg/ddz178. Hum Mol Genet. 2019. PMID: 31348488 Free PMC article. Review.
-
Animal-to-Animal Variability in Neuromodulation and Circuit Function.Cold Spring Harb Symp Quant Biol. 2014;79:21-8. doi: 10.1101/sqb.2014.79.024828. Epub 2015 Apr 15. Cold Spring Harb Symp Quant Biol. 2014. PMID: 25876630 Free PMC article. Review.
Cited by
-
Neuronal Intrinsic Physiology Changes During Development of a Learned Behavior.eNeuro. 2017 Oct 20;4(5):ENEURO.0297-17.2017. doi: 10.1523/ENEURO.0297-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 29062887 Free PMC article.
-
NPAS4 supports cocaine-conditioned cues in rodents by controlling the cell type-specific activation balance in the nucleus accumbens.Nat Commun. 2024 Aug 8;15(1):5971. doi: 10.1038/s41467-024-50099-1. Nat Commun. 2024. PMID: 39117647 Free PMC article.
-
Generation and customization of biosynthetic excitable tissues for electrophysiological studies and cell-based therapies.Nat Protoc. 2018 May;13(5):927-945. doi: 10.1038/nprot.2018.016. Epub 2018 Apr 5. Nat Protoc. 2018. PMID: 29622805 Free PMC article.
-
Npas4: Linking Neuronal Activity to Memory.Trends Neurosci. 2016 Apr;39(4):264-275. doi: 10.1016/j.tins.2016.02.003. Epub 2016 Mar 14. Trends Neurosci. 2016. PMID: 26987258 Free PMC article. Review.
-
Unsupervised restoration of a complex learned behavior after large-scale neuronal perturbation.Nat Neurosci. 2024 Jun;27(6):1176-1186. doi: 10.1038/s41593-024-01630-6. Epub 2024 Apr 29. Nat Neurosci. 2024. PMID: 38684893
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
