Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse
- PMID: 23633490
- PMCID: PMC3702668
- DOI: 10.1158/0008-5472.CAN-12-4521
Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse
Abstract
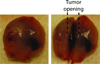
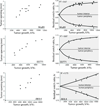
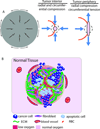
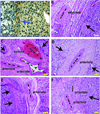
The stress harbored by the solid phase of tumors is known as solid stress. Solid stress can be either applied externally by the surrounding normal tissue or induced by the tumor itself due to its growth. Fluid pressure is the isotropic stress exerted by the fluid phase. We recently showed that growth-induced solid stress is on the order of 1.3 to 13.0 kPa (10-100 mmHg)--high enough to cause compression of fragile blood vessels, resulting in poor perfusion and hypoxia. However, the evolution of growth-induced stress with tumor progression and its effect on cancer cell proliferation in vivo is not understood. To this end, we developed a mathematical model for tumor growth that takes into account all three types of stresses: growth-induced stress, externally applied stress, and fluid pressure. First, we conducted in vivo experiments and found that growth-induced stress is related to tumor volume through a biexponential relationship. Then, we incorporated this information into our mathematical model and showed that due to the evolution of growth-induced stress, total solid stress levels are higher in the tumor interior and lower in the periphery. Elevated compressive solid stress in the interior of the tumor is sufficient to cause the collapse of blood vessels and results in a lower growth rate of cancer cells compared with the periphery, independently from that caused by the lack of nutrients due to vessel collapse. Furthermore, solid stress in the periphery of the tumor causes blood vessels in the surrounding normal tissue to deform to elliptical shapes. We present histologic sections of human cancers that show such vessel deformations. Finally, we found that fluid pressure increases with tumor growth due to increased vascular permeability and lymphatic impairment, and is governed by the microvascular pressure. Crucially, fluid pressure does not cause vessel compression of tumor vessels.
©2013 AACR.
Figures



Similar articles
-
The effect of interstitial pressure on tumor growth: coupling with the blood and lymphatic vascular systems.J Theor Biol. 2013 Mar 7;320:131-51. doi: 10.1016/j.jtbi.2012.11.031. Epub 2012 Dec 7. J Theor Biol. 2013. PMID: 23220211 Free PMC article.
-
The Solid Mechanics of Cancer and Strategies for Improved Therapy.J Biomech Eng. 2017 Feb 1;139(2):10.1115/1.4034991. doi: 10.1115/1.4034991. J Biomech Eng. 2017. PMID: 27760260 Free PMC article. Review.
-
Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors.Proc Natl Acad Sci U S A. 2012 Sep 18;109(38):15101-8. doi: 10.1073/pnas.1213353109. Epub 2012 Aug 29. Proc Natl Acad Sci U S A. 2012. PMID: 22932871 Free PMC article.
-
Interstitial stress and fluid pressure within a growing tumor.Ann Biomed Eng. 2003 Mar;31(3):327-35. doi: 10.1114/1.1554923. Ann Biomed Eng. 2003. PMID: 12680730
-
The role of mechanical forces in tumor growth and therapy.Annu Rev Biomed Eng. 2014 Jul 11;16:321-46. doi: 10.1146/annurev-bioeng-071813-105259. Annu Rev Biomed Eng. 2014. PMID: 25014786 Free PMC article. Review.
Cited by
-
Biomechanical modelling of spinal tumour anisotropic growth.Proc Math Phys Eng Sci. 2020 Jun;476(2238):20190364. doi: 10.1098/rspa.2019.0364. Epub 2020 Jun 3. Proc Math Phys Eng Sci. 2020. PMID: 32831581 Free PMC article.
-
Stress-mediated progression of solid tumors: effect of mechanical stress on tissue oxygenation, cancer cell proliferation, and drug delivery.Biomech Model Mechanobiol. 2015 Nov;14(6):1391-402. doi: 10.1007/s10237-015-0682-0. Epub 2015 May 13. Biomech Model Mechanobiol. 2015. PMID: 25968141 Free PMC article.
-
The biophysics of cancer: emerging insights from micro- and nanoscale tools.Adv Nanobiomed Res. 2022 Jan;2(1):2100056. doi: 10.1002/anbr.202100056. Epub 2021 Nov 23. Adv Nanobiomed Res. 2022. PMID: 35156093 Free PMC article.
-
Non-Invasive Imaging of Normalized Solid Stress in Cancers in Vivo.IEEE J Transl Eng Health Med. 2019 Sep 13;7:4300209. doi: 10.1109/JTEHM.2019.2932059. eCollection 2019. IEEE J Transl Eng Health Med. 2019. PMID: 32309062 Free PMC article.
-
Understanding Drug Resistance in Breast Cancer with Mathematical Oncology.Curr Breast Cancer Rep. 2014 Jun 1;6(2):110-120. doi: 10.1007/s12609-014-0143-2. Curr Breast Cancer Rep. 2014. PMID: 24891927 Free PMC article.
References
-
- Skalak R, Zargaryan S, Jain RK, Netti PA, Hoger A. Compatibility and the genesis of residual stress by volumetric growth. J Math Biol. 1996;34(8):889–914. - PubMed
-
- Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15(8):778–783. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA126642/CA/NCI NIH HHS/United States
- R01-CA085140/CA/NCI NIH HHS/United States
- R01-CA098706/CA/NCI NIH HHS/United States
- R01 CA098706/CA/NCI NIH HHS/United States
- R01 CA085140/CA/NCI NIH HHS/United States
- K12 CA090354/CA/NCI NIH HHS/United States
- P01-CA080124/CA/NCI NIH HHS/United States
- R24 CA085140/CA/NCI NIH HHS/United States
- R01-CA096915/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- R01 CA163815/CA/NCI NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- 2K12CA090354-11/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

