Molecular ageing of alpha- and Beta-synucleins: protein damage and repair mechanisms
- PMID: 23630590
- PMCID: PMC3632608
- DOI: 10.1371/journal.pone.0061442
Molecular ageing of alpha- and Beta-synucleins: protein damage and repair mechanisms
Abstract
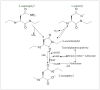
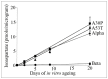
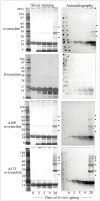
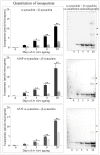
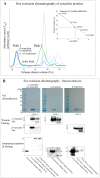
Abnormal α-synuclein aggregates are hallmarks of a number of neurodegenerative diseases. Alpha synuclein and β-synucleins are susceptible to post-translational modification as isoaspartate protein damage, which is regulated in vivo by the action of the repair enzyme protein L-isoaspartyl O-methyltransferase (PIMT). We aged in vitro native α-synuclein, the α-synuclein familial mutants A30P and A53T that give rise to Parkinsonian phenotypes, and β-synuclein, at physiological pH and temperature for a time course of up to 20 days. Resolution of native α-synuclein and β-synuclein by two dimensional techniques showed the accumulation of a number of post-translationally modified forms of both proteins. The levels of isoaspartate formed over the 20 day time course were quantified by exogenous methylation with PIMT using S-Adenosyl-L-[(3)H-methyl]methionine as a methyl donor, and liquid scintillation counting of liberated (3)H-methanol. All α-synuclein proteins accumulated isoaspartate at ∼1% of molecules/day, ∼20 times faster than for β-synuclein. This disparity between rates of isoaspartate was confirmed by exogenous methylation of synucleins by PIMT, protein resolution by one-dimensional denaturing gel electrophoresis, and visualisation of (3)H-methyl esters by autoradiography. Protein silver staining and autoradiography also revealed that α-synucleins accumulated stable oligomers that were resistant to denaturing conditions, and which also contained isoaspartate. Co-incubation of approximately equimolar β-synuclein with α-synuclein resulted in a significant reduction of isoaspartate formed in all α-synucleins after 20 days of ageing. Co-incubated α- and β-synucleins, or α, or β synucleins alone, were resolved by non-denaturing size exclusion chromatography and all formed oligomers of ∼57.5 kDa; consistent with tetramerization. Direct association of α-synuclein with β-synuclein in column fractions or from in vitro ageing co-incubations was demonstrated by their co-immunoprecipitation. These results provide an insight into the molecular differences between α- and β-synucleins during ageing, and highlight the susceptibility of α-synuclein to protein damage, and the potential protective role of β-synuclein.
Conflict of interest statement
Figures



Similar articles
-
Considerations in the identification of endogenous substrates for protein L-isoaspartyl methyltransferase: the case of synuclein.PLoS One. 2012;7(8):e43288. doi: 10.1371/journal.pone.0043288. Epub 2012 Aug 14. PLoS One. 2012. PMID: 22905247 Free PMC article.
-
Protein repair in the brain, proteomic analysis of endogenous substrates for protein L-isoaspartyl methyltransferase in mouse brain.J Biol Chem. 2006 Nov 3;281(44):33802-13. doi: 10.1074/jbc.M606958200. Epub 2006 Sep 7. J Biol Chem. 2006. PMID: 16959769
-
Isoaspartate, carbamoyl phosphate synthase-1, and carbonic anhydrase-III as biomarkers of liver injury.Biochem Biophys Res Commun. 2015 Mar 13;458(3):626-631. doi: 10.1016/j.bbrc.2015.01.158. Epub 2015 Feb 13. Biochem Biophys Res Commun. 2015. PMID: 25684186 Free PMC article.
-
Biological significance of isoaspartate and its repair system.Biol Pharm Bull. 2005 Sep;28(9):1590-6. doi: 10.1248/bpb.28.1590. Biol Pharm Bull. 2005. PMID: 16141521 Review.
-
Isoaspartate formation and neurodegeneration in Alzheimer's disease.Arch Biochem Biophys. 2000 Sep 15;381(2):225-34. doi: 10.1006/abbi.2000.1955. Arch Biochem Biophys. 2000. PMID: 11032409 Review.
Cited by
-
β-Synuclein: An Enigmatic Protein with Diverse Functionality.Biomolecules. 2022 Jan 16;12(1):142. doi: 10.3390/biom12010142. Biomolecules. 2022. PMID: 35053291 Free PMC article. Review.
-
Differentiated Neurons Are More Vulnerable to Organophosphate and Carbamate Neurotoxicity than Undifferentiated Neurons Due to the Induction of Redox Stress and Accumulate Oxidatively-Damaged Proteins.Brain Sci. 2023 Apr 26;13(5):728. doi: 10.3390/brainsci13050728. Brain Sci. 2023. PMID: 37239200 Free PMC article.
-
Implications of DNA Methylation in Parkinson's Disease.Front Mol Neurosci. 2017 Jul 18;10:225. doi: 10.3389/fnmol.2017.00225. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28769760 Free PMC article. Review.
-
Ceruloplasmin Deamidation in Neurodegeneration: From Loss to Gain of Function.Int J Mol Sci. 2021 Jan 11;22(2):663. doi: 10.3390/ijms22020663. Int J Mol Sci. 2021. PMID: 33440850 Free PMC article. Review.
-
PCMT1 regulates the migration, invasion, and apoptosis of prostate cancer through modulating the PI3K/AKT/GSK-3β pathway.Aging (Albany NY). 2023 Oct 27;15(20):11654-11671. doi: 10.18632/aging.205152. Epub 2023 Oct 27. Aging (Albany NY). 2023. PMID: 37899170 Free PMC article.
References
-
- Jakes R, Spillantini MG, Goedert M (1994) Identification of two distinct synucleins from human brain. FEBS Lett 345: 27–32. - PubMed
-
- Goedert M (2001) Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2: 492–501. - PubMed
-
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047. - PubMed
-
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, et al. (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet 18: 106–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

