Conservation and de novo acquisition of dosage compensation on newly evolved sex chromosomes in Drosophila
- PMID: 23630075
- PMCID: PMC3650223
- DOI: 10.1101/gad.215426.113
Conservation and de novo acquisition of dosage compensation on newly evolved sex chromosomes in Drosophila
Abstract
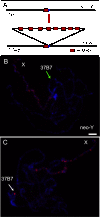
Dosage compensation has arisen in response to the evolution of distinct male (XY) and female (XX) karyotypes. In Drosophila melanogaster, the MSL complex increases male X transcription approximately twofold. X-specific targeting is thought to occur through sequence-dependent binding to chromatin entry sites (CESs), followed by spreading in cis to active genes. We tested this model by asking how newly evolving sex chromosome arms in Drosophila miranda acquired dosage compensation. We found evidence for the creation of new CESs, with the analogous sequence and spacing as in D. melanogaster, providing strong support for the spreading model in the establishment of dosage compensation.
Figures
Similar articles
-
Contingency in the convergent evolution of a regulatory network: Dosage compensation in Drosophila.PLoS Biol. 2019 Feb 11;17(2):e3000094. doi: 10.1371/journal.pbio.3000094. eCollection 2019 Feb. PLoS Biol. 2019. PMID: 30742611 Free PMC article.
-
Sex-specific embryonic gene expression in species with newly evolved sex chromosomes.PLoS Genet. 2014 Feb 13;10(2):e1004159. doi: 10.1371/journal.pgen.1004159. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24550743 Free PMC article.
-
Dosage compensation regulatory proteins and the evolution of sex chromosomes in Drosophila.Genetics. 1996 Oct;144(2):705-13. doi: 10.1093/genetics/144.2.705. Genetics. 1996. PMID: 8889531 Free PMC article.
-
Recent progress and open questions in Drosophila dosage compensation.Fly (Austin). 2015;9(1):29-35. doi: 10.1080/19336934.2015.1074786. Fly (Austin). 2015. PMID: 26213294 Free PMC article. Review.
-
Sex and the single chromosome.Adv Genet. 2002;46:1-24. doi: 10.1016/s0065-2660(02)46002-6. Adv Genet. 2002. PMID: 11931222 Review.
Cited by
-
Sex chromosome evolution: life, death and repetitive DNA.Fly (Austin). 2014;8(4):197-9. doi: 10.1080/19336934.2015.1024395. Epub 2015 Mar 9. Fly (Austin). 2014. PMID: 25751570 Free PMC article.
-
X-chromosome target specificity diverged between dosage compensation mechanisms of two closely related Caenorhabditis species.Elife. 2023 Mar 23;12:e85413. doi: 10.7554/eLife.85413. Elife. 2023. PMID: 36951246 Free PMC article.
-
Contingency in the convergent evolution of a regulatory network: Dosage compensation in Drosophila.PLoS Biol. 2019 Feb 11;17(2):e3000094. doi: 10.1371/journal.pbio.3000094. eCollection 2019 Feb. PLoS Biol. 2019. PMID: 30742611 Free PMC article.
-
Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in Drosophila.PLoS Genet. 2015 Jun 26;11(6):e1005331. doi: 10.1371/journal.pgen.1005331. eCollection 2015 Jun. PLoS Genet. 2015. PMID: 26114585 Free PMC article.
-
The CLAMP protein links the MSL complex to the X chromosome during Drosophila dosage compensation.Genes Dev. 2013 Jul 15;27(14):1551-6. doi: 10.1101/gad.214585.113. Genes Dev. 2013. PMID: 23873939 Free PMC article.
References
-
- Bachtrog D, Charlesworth B 2002. Reduced adaptation of a non-recombining neo-Y chromosome. Nature 416: 323–326 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
