Detection of G protein-selective G protein-coupled receptor (GPCR) conformations in live cells
- PMID: 23629648
- PMCID: PMC3682522
- DOI: 10.1074/jbc.M113.464065
Detection of G protein-selective G protein-coupled receptor (GPCR) conformations in live cells
Abstract
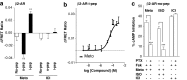
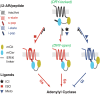
Although several recent studies have reported that GPCRs adopt multiple conformations, it remains unclear how subtle conformational changes are translated into divergent downstream responses. In this study, we report on a novel class of FRET-based sensors that can detect the ligand/mutagenic stabilization of GPCR conformations that promote interactions with G proteins in live cells. These sensors rely on the well characterized interaction between a GPCR and the C terminus of a Gα subunit. We use these sensors to elucidate the influence of the highly conserved (E/D)RY motif on GPCR conformation. Specifically, Glu/Asp but not Arg mutants of the (E/D)RY motif are known to enhance basal GPCR signaling. Hence, it is unclear whether ionic interactions formed by the (E/D)RY motif (ionic lock) are necessary to stabilize basal GPCR states. We find that mutagenesis of the β2-AR (E/D)RY ionic lock enhances interaction with Gs. However, only Glu/Asp but not Arg mutants increase G protein activation. In contrast, mutagenesis of the opsin (E/D)RY ionic lock does not alter its interaction with transducin. Instead, opsin-specific ionic interactions centered on residue Lys-296 are both necessary and sufficient to promote interactions with transducin. Effective suppression of β2-AR basal activity by inverse agonist ICI 118,551 requires ionic interactions formed by the (E/D)RY motif. In contrast, the inverse agonist metoprolol suppresses interactions with Gs and promotes Gi binding, with concomitant pertussis toxin-sensitive inhibition of adenylyl cyclase activity. Taken together, these studies validate the use of the new FRET sensors while revealing distinct structural mechanisms for ligand-dependent GPCR function.
Keywords: (E/D)RY Motif; 7-Helix Receptor; Cell Signaling; Fret; Functional Selectivity; G Protein-coupled Receptors (GPCR); G Proteins.
Figures


Similar articles
-
Tyrosine 308 is necessary for ligand-directed Gs protein-biased signaling of β2-adrenoceptor.J Biol Chem. 2014 Jul 11;289(28):19351-63. doi: 10.1074/jbc.M114.558882. Epub 2014 May 15. J Biol Chem. 2014. PMID: 24831005 Free PMC article.
-
Molecular dynamics simulations of the effect of the G-protein and diffusible ligands on the β2-adrenergic receptor.J Mol Biol. 2011 Dec 9;414(4):611-23. doi: 10.1016/j.jmb.2011.10.015. Epub 2011 Oct 20. J Mol Biol. 2011. PMID: 22037586
-
Functional differences between full and partial agonists: evidence for ligand-specific receptor conformations.J Pharmacol Exp Ther. 2001 Jun;297(3):1218-26. J Pharmacol Exp Ther. 2001. PMID: 11356949
-
Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function.Pharmacol Ther. 2004 Jul;103(1):21-80. doi: 10.1016/j.pharmthera.2004.05.002. Pharmacol Ther. 2004. PMID: 15251227 Review.
-
Structural features of β2 adrenergic receptor: crystal structures and beyond.Mol Cells. 2015;38(2):105-11. doi: 10.14348/molcells.2015.2301. Epub 2014 Dec 24. Mol Cells. 2015. PMID: 25537861 Free PMC article. Review.
Cited by
-
Structural Elements in the Gαs and Gαq C Termini That Mediate Selective G Protein-coupled Receptor (GPCR) Signaling.J Biol Chem. 2016 Aug 19;291(34):17929-40. doi: 10.1074/jbc.M116.735720. Epub 2016 Jun 21. J Biol Chem. 2016. PMID: 27330078 Free PMC article.
-
G Protein-selective GPCR Conformations Measured Using FRET Sensors in a Live Cell Suspension Fluorometer Assay.J Vis Exp. 2016 Sep 10;(115):54696. doi: 10.3791/54696. J Vis Exp. 2016. PMID: 27684955 Free PMC article.
-
Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks.Chem Rev. 2018 Dec 26;118(24):11707-11794. doi: 10.1021/acs.chemrev.8b00333. Epub 2018 Dec 14. Chem Rev. 2018. PMID: 30550275 Free PMC article. Review.
-
Spatial encoding of GPCR signaling in the nervous system.Curr Opin Cell Biol. 2019 Apr;57:83-89. doi: 10.1016/j.ceb.2018.12.006. Epub 2019 Jan 29. Curr Opin Cell Biol. 2019. PMID: 30708280 Free PMC article. Review.
-
ER/K linked GPCR-G protein fusions systematically modulate second messenger response in cells.Sci Rep. 2017 Aug 10;7(1):7749. doi: 10.1038/s41598-017-08029-3. Sci Rep. 2017. PMID: 28798477 Free PMC article.
References
-
- Vilardaga J. P., Steinmeyer R., Harms G. S., Lohse M. J. (2005) Molecular basis of inverse agonism in a G protein-coupled receptor. Nat. Chem. Biol. 1, 25–28 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

