Wnt7a regulates multiple steps of neurogenesis
- PMID: 23629626
- PMCID: PMC3700117
- DOI: 10.1128/MCB.00325-13
Wnt7a regulates multiple steps of neurogenesis
Abstract
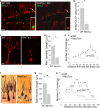
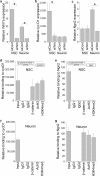
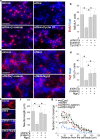
Although Wnt7a has been implicated in axon guidance and synapse formation, investigations of its role in the early steps of neurogenesis have just begun. We show here that Wnt7a is essential for neural stem cell self-renewal and neural progenitor cell cycle progression in adult mouse brains. Loss of Wnt7a expression dramatically reduced the neural stem cell population and increased the rate of cell cycle exit in neural progenitors in the hippocampal dentate gyrus of adult mice. Furthermore, Wnt7a is important for neuronal differentiation and maturation. Loss of Wnt7a expression led to a substantial decrease in the number of newborn neurons in the hippocampal dentate gyrus. Wnt7a(-/-) dentate granule neurons exhibited dramatically impaired dendritic development. Moreover, Wnt7a activated β-catenin and its downstream target genes to regulate neural stem cell proliferation and differentiation. Wnt7a stimulated neural stem cell proliferation by activating the β-catenin-cyclin D1 pathway and promoted neuronal differentiation and maturation by inducing the β-catenin-neurogenin 2 pathway. Thus, Wnt7a exercised critical control over multiple steps of neurogenesis by regulating genes involved in both cell cycle control and neuronal differentiation.
Figures
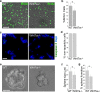
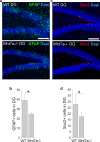
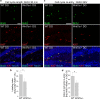
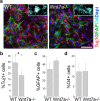
Similar articles
-
Inducible and conditional deletion of extracellular signal-regulated kinase 5 disrupts adult hippocampal neurogenesis.J Biol Chem. 2012 Jul 6;287(28):23306-17. doi: 10.1074/jbc.M112.344762. Epub 2012 May 29. J Biol Chem. 2012. PMID: 22645146 Free PMC article.
-
Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal.Nat Cell Biol. 2010 Jan;12(1):31-40; sup pp 1-9. doi: 10.1038/ncb2001. Epub 2009 Dec 13. Nat Cell Biol. 2010. PMID: 20010817 Free PMC article.
-
Diverse roles for Wnt7a in ventral midbrain neurogenesis and dopaminergic axon morphogenesis.Stem Cells Dev. 2014 Sep 1;23(17):1991-2003. doi: 10.1089/scd.2014.0166. Epub 2014 Jun 30. Stem Cells Dev. 2014. PMID: 24803261
-
Neural stem cell of the hippocampus: development, physiology regulation, and dysfunction in disease.Curr Top Dev Biol. 2014;107:183-206. doi: 10.1016/B978-0-12-416022-4.00007-X. Curr Top Dev Biol. 2014. PMID: 24439807 Review.
-
Adult neurogenesis in the mammalian dentate gyrus.Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review.
Cited by
-
Complementary Wnt Sources Regulate Lymphatic Vascular Development via PROX1-Dependent Wnt/β-Catenin Signaling.Cell Rep. 2018 Oct 16;25(3):571-584.e5. doi: 10.1016/j.celrep.2018.09.049. Cell Rep. 2018. PMID: 30332639 Free PMC article.
-
Wnt signaling and the control of human stem cell fate.Stem Cell Rev Rep. 2014 Apr;10(2):207-29. doi: 10.1007/s12015-013-9486-8. Stem Cell Rev Rep. 2014. PMID: 24323281 Review.
-
mTOR regulates brain morphogenesis by mediating GSK3 signaling.Development. 2014 Nov;141(21):4076-86. doi: 10.1242/dev.108282. Epub 2014 Oct 1. Development. 2014. PMID: 25273085 Free PMC article.
-
A multi-resource data integration approach: identification of candidate genes regulating cell proliferation during neocortical development.Front Neurosci. 2014 Aug 21;8:257. doi: 10.3389/fnins.2014.00257. eCollection 2014. Front Neurosci. 2014. PMID: 25191221 Free PMC article.
-
COMMD10 Is Essential for Neural Plate Development during Embryogenesis.J Dev Biol. 2023 Mar 16;11(1):13. doi: 10.3390/jdb11010013. J Dev Biol. 2023. PMID: 36976102 Free PMC article.
References
-
- Gage FH. 2000. Mammalian neural stem cells. Science 287:1433–1438 - PubMed
-
- Chenn A, Walsh CA. 2002. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297:365–369 - PubMed
-
- Lee SM, Tole S, Grove E, McMahon AP. 2000. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 127:457–467 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
