IL-6 activated integrated BATF/IRF4 functions in lymphocytes are T-bet-independent and reversed by subcutaneous immunotherapy
- PMID: 23628948
- PMCID: PMC3639449
- DOI: 10.1038/srep01754
IL-6 activated integrated BATF/IRF4 functions in lymphocytes are T-bet-independent and reversed by subcutaneous immunotherapy
Abstract
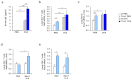
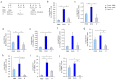
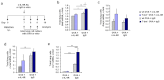
IL-6 plays a central role in supporting pathological T(H2) and T(H17) cell development and inhibiting the protective T regulatory cells in allergic asthma. T(H17) cells have been demonstrated to regulate allergic asthma in general and T-bet-deficiency-induced asthma in particular. Here we found an inverse correlation between T-bet and Il-6 mRNA expression in asthmatic children. Moreover, experimental subcutaneous immunotherapy (SIT) in T-bet((-/-)) mice inhibited IL-6, IL-21R and lung T(H17) cells in a setting of asthma. Finally, local delivery of an anti-IL-6R antibody in T-bet((-/-)) mice resulted in the resolution of this allergic trait. Noteworthy, BATF, crucial for the immunoglobulin-class-switch and T(H2),T(H17) development, was found down-regulated in the lungs of T-bet((-/-)) mice after SIT and after treatment with anti-IL-6R antibody, indicating a critical role of IL-6 in controlling BATF/IRF4 integrated functions in T(H2), T(H17) cells and B cells also in a T-bet independent fashion in allergic asthma.
Figures





Similar articles
-
The activating protein 1 transcription factor basic leucine zipper transcription factor, ATF-like (BATF), regulates lymphocyte- and mast cell-driven immune responses in the setting of allergic asthma.J Allergy Clin Immunol. 2014 Jan;133(1):198-206.e1-9. doi: 10.1016/j.jaci.2013.09.049. Epub 2013 Nov 28. J Allergy Clin Immunol. 2014. PMID: 24290279
-
Elevated interferon regulatory factor 4 levels in patients with allergic asthma.J Asthma. 2012 Jun;49(5):441-9. doi: 10.3109/02770903.2012.674998. Epub 2012 Apr 19. J Asthma. 2012. PMID: 22515488
-
The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells.Nat Immunol. 2014 Apr;15(4):373-83. doi: 10.1038/ni.2834. Epub 2014 Mar 2. Nat Immunol. 2014. PMID: 24584090 Free PMC article.
-
Pathological role of IL-6 in the experimental allergic bronchial asthma in mice.Clin Rev Allergy Immunol. 2005 Jun;28(3):257-70. doi: 10.1385/CRIAI:28:3:257. Clin Rev Allergy Immunol. 2005. PMID: 16129910 Review.
-
The transcription factor BATF modulates cytokine-mediated responses in T cells.Cytokine Growth Factor Rev. 2016 Aug;30:39-45. doi: 10.1016/j.cytogfr.2016.03.004. Epub 2016 Mar 6. Cytokine Growth Factor Rev. 2016. PMID: 26970726 Review.
Cited by
-
Discovering in vivo cytokine-eQTL interactions from a lupus clinical trial.Genome Biol. 2018 Oct 19;19(1):168. doi: 10.1186/s13059-018-1560-8. Genome Biol. 2018. PMID: 30340504 Free PMC article. Clinical Trial.
-
Increased expression of the Th17-IL-6R/pSTAT3/BATF/RorγT-axis in the tumoural region of adenocarcinoma as compared to squamous cell carcinoma of the lung.Sci Rep. 2014 Dec 10;4:7396. doi: 10.1038/srep07396. Sci Rep. 2014. PMID: 25491772 Free PMC article. Clinical Trial.
-
Increased BATF expression is associated with the severity of liver damage in patients with chronic hepatitis B.Clin Exp Med. 2018 May;18(2):263-272. doi: 10.1007/s10238-017-0480-3. Epub 2017 Nov 21. Clin Exp Med. 2018. PMID: 29164410
-
Factors involved in CLL pathogenesis and cell survival are disrupted by differentiation of CLL B-cells into antibody-secreting cells.Oncotarget. 2015 Jul 30;6(21):18484-503. doi: 10.18632/oncotarget.3941. Oncotarget. 2015. PMID: 26050196 Free PMC article.
-
Tryptophan metabolite-regulated Treg responses contribute to attenuation of airway inflammation during specific immunotherapy in a mouse asthma model.Hum Vaccin Immunother. 2020 Aug 2;16(8):1891-1899. doi: 10.1080/21645515.2019.1698900. Epub 2020 Jan 17. Hum Vaccin Immunother. 2020. PMID: 31951781 Free PMC article.
References
-
- Holgate S. T. & Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol 8, 218–230 (2008). - PubMed
-
- Akdis C. A. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med 18, 736–749 (2012). - PubMed
-
- Akdis M. & Akdis C. A. Therapeutic manipulation of immune tolerance in allergic disease. Nat Rev Drug Discov 8, 645–660 (2009). - PubMed
-
- Durham S. R. et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol 125, 131–138 e131–137 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

