Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning
- PMID: 23624372
- PMCID: PMC4197975
- DOI: 10.1038/nature12157
Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning
Abstract
The ability of signalling proteins to traverse tissues containing tightly packed cells is of fundamental importance for cell specification and tissue development; however, how this is achieved at a cellular level remains poorly understood. For more than a century, the vertebrate limb bud has served as a model for studying cell signalling during embryonic development. Here we optimize single-cell real-time imaging to delineate the cellular mechanisms for how signalling proteins, such as sonic hedgehog (SHH), that possess membrane-bound covalent lipid modifications traverse long distances within the vertebrate limb bud in vivo. By directly imaging SHH ligand production under native regulatory control in chick (Gallus gallus) embryos, our findings show that SHH is unexpectedly produced in the form of a particle that remains associated with the cell via long cytoplasmic extensions that span several cell diameters. We show that these cellular extensions are a specialized class of actin-based filopodia with novel cytoskeletal features that have not been previously described. Notably, particles containing SHH travel along these extensions with a net anterograde movement within the field of SHH cell signalling. We further show that in SHH-responding cells, specific subsets of SHH co-receptors, including cell adhesion molecule downregulated by oncogenes (CDO) and brother of CDO (BOC), actively distribute and co-localize in specific micro-domains within filopodial extensions, far from the cell body. Stabilized interactions are formed between filopodia containing SHH ligand and those containing co-receptors over a long range. These results suggest that contact-mediated release propagated by specialized filopodia contributes to the delivery of SHH at a distance. Together, these studies identify an important mode of communication between cells that considerably extends our understanding of ligand movement and reception during vertebrate tissue patterning.
Figures
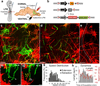
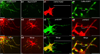
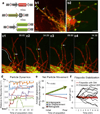
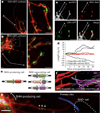
Comment in
-
Cytonemes extend their reach.EMBO J. 2013 Jun 12;32(12):1658-9. doi: 10.1038/emboj.2013.115. Epub 2013 May 14. EMBO J. 2013. PMID: 23673359 Free PMC article.
-
Filopodia: the cellular quills of hedgehog signaling?Dev Cell. 2013 May 28;25(4):328-30. doi: 10.1016/j.devcel.2013.05.008. Dev Cell. 2013. PMID: 23725760
Similar articles
-
Filopodia: the cellular quills of hedgehog signaling?Dev Cell. 2013 May 28;25(4):328-30. doi: 10.1016/j.devcel.2013.05.008. Dev Cell. 2013. PMID: 23725760
-
Cytoneme delivery of Sonic Hedgehog from ligand-producing cells requires Myosin 10 and a Dispatched-BOC/CDON co-receptor complex.Elife. 2021 Feb 11;10:e61432. doi: 10.7554/eLife.61432. Elife. 2021. PMID: 33570491 Free PMC article.
-
Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function.Dev Biol. 2001 Aug 15;236(2):421-35. doi: 10.1006/dbio.2001.0346. Dev Biol. 2001. PMID: 11476582
-
How the embryo makes a limb: determination, polarity and identity.J Anat. 2015 Oct;227(4):418-30. doi: 10.1111/joa.12361. Epub 2015 Aug 7. J Anat. 2015. PMID: 26249743 Free PMC article. Review.
-
Specialized filopodia: at the 'tip' of morphogen transport and vertebrate tissue patterning.Curr Opin Genet Dev. 2014 Aug;27:67-73. doi: 10.1016/j.gde.2014.03.013. Epub 2014 Jun 5. Curr Opin Genet Dev. 2014. PMID: 24907447 Free PMC article. Review.
Cited by
-
Basal epidermis collective migration and local Sonic hedgehog signaling promote skeletal branching morphogenesis in zebrafish fins.Dev Biol. 2021 Sep;477:177-190. doi: 10.1016/j.ydbio.2021.04.010. Epub 2021 May 24. Dev Biol. 2021. PMID: 34038742 Free PMC article.
-
Direct Cell-Cell Communication via Membrane Pores, Gap Junction Channels, and Tunneling Nanotubes: Medical Relevance of Mitochondrial Exchange.Int J Mol Sci. 2022 May 30;23(11):6133. doi: 10.3390/ijms23116133. Int J Mol Sci. 2022. PMID: 35682809 Free PMC article. Review.
-
The chaperone ERp29 is required for tunneling nanotube formation by stabilizing MSec.J Biol Chem. 2019 May 3;294(18):7177-7193. doi: 10.1074/jbc.RA118.005659. Epub 2019 Mar 15. J Biol Chem. 2019. PMID: 30877198 Free PMC article.
-
Fixation of Embryonic Mouse Tissue for Cytoneme Analysis.J Vis Exp. 2022 Jun 16;(184):10.3791/64100. doi: 10.3791/64100. J Vis Exp. 2022. PMID: 35786607 Free PMC article.
-
Loss of the Heparan Sulfate Proteoglycan Glypican5 Facilitates Long-Range Sonic Hedgehog Signaling.Stem Cells. 2019 Jul;37(7):899-909. doi: 10.1002/stem.3018. Epub 2019 May 13. Stem Cells. 2019. PMID: 30977233 Free PMC article.
References
-
- Zhu AJ, Scott MP. Incredible journey: how do developmental signals travel through tissue? Genes Dev. 2004;18:2985–2997. - PubMed
-
- Niswander L. Pattern formation: old models out on a limb. Nat Rev Genet. 2003;4:133–143. - PubMed
-
- Hsiung F, Ramirez-Weber F-A, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. - PubMed
-
- Ramírez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

