ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation
- PMID: 23620409
- PMCID: PMC3687028
- DOI: 10.1158/1078-0432.CCR-12-3408
ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation
Abstract
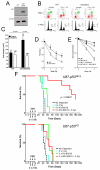
Purpose: Glioblastoma multiforme (GBM) is the most lethal form of brain cancer with a median survival of only 12 to 15 months. Current standard treatment consists of surgery followed by chemoradiation. The poor survival of patients with GBM is due to aggressive tumor invasiveness, an inability to remove all tumor tissue, and an innate tumor chemo- and radioresistance. Ataxia-telangiectasia mutated (ATM) is an excellent target for radiosensitizing GBM because of its critical role in regulating the DNA damage response and p53, among other cellular processes. As a first step toward this goal, we recently showed that the novel ATM kinase inhibitor KU-60019 reduced migration, invasion, and growth, and potently radiosensitized human glioma cells in vitro.
Experimental design: Using orthotopic xenograft models of GBM, we now show that KU-60019 is also an effective radiosensitizer in vivo. Human glioma cells expressing reporter genes for monitoring tumor growth and dispersal were grown intracranially, and KU-60019 was administered intratumorally by convection-enhanced delivery or osmotic pump.
Results: Our results show that the combined effect of KU-60019 and radiation significantly increased survival of mice 2- to 3-fold over controls. Importantly, we show that glioma with mutant p53 is much more sensitive to KU-60019 radiosensitization than genetically matched wild-type glioma.
Conclusions: Taken together, our results suggest that an ATM kinase inhibitor may be an effective radiosensitizer and adjuvant therapy for patients with mutant p53 brain cancers.
Figures

Similar articles
-
Dynamic inhibition of ATM kinase provides a strategy for glioblastoma multiforme radiosensitization and growth control.Cell Cycle. 2012 Mar 15;11(6):1167-73. doi: 10.4161/cc.11.6.19576. Epub 2012 Mar 15. Cell Cycle. 2012. PMID: 22370485 Free PMC article.
-
Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion.Mol Cancer Ther. 2009 Oct;8(10):2894-902. doi: 10.1158/1535-7163.MCT-09-0519. Epub 2009 Oct 6. Mol Cancer Ther. 2009. PMID: 19808981 Free PMC article.
-
The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models.Sci Adv. 2018 Jun 20;4(6):eaat1719. doi: 10.1126/sciadv.aat1719. eCollection 2018 Jun. Sci Adv. 2018. PMID: 29938225 Free PMC article.
-
The efficacy and toxicity of ATM inhibition in glioblastoma initiating cells-driven tumor models.Crit Rev Oncol Hematol. 2019 Jun;138:214-222. doi: 10.1016/j.critrevonc.2019.04.015. Epub 2019 Apr 21. Crit Rev Oncol Hematol. 2019. PMID: 31092378 Review.
-
ATM as a target for novel radiosensitizers.Semin Radiat Oncol. 2001 Oct;11(4):316-27. doi: 10.1053/srao.2001.26030. Semin Radiat Oncol. 2001. PMID: 11677656 Review.
Cited by
-
Aberrant ATM signaling and homology-directed DNA repair as a vulnerability of p53-mutant GBM to AZD1390-mediated radiosensitization.Sci Transl Med. 2024 Feb 14;16(734):eadj5962. doi: 10.1126/scitranslmed.adj5962. Epub 2024 Feb 14. Sci Transl Med. 2024. PMID: 38354228 Free PMC article.
-
Combination Therapy with Reovirus and ATM Inhibitor Enhances Cell Death and Virus Replication in Canine Melanoma.Mol Ther Oncolytics. 2019 Aug 28;15:49-59. doi: 10.1016/j.omto.2019.08.003. eCollection 2019 Dec 20. Mol Ther Oncolytics. 2019. PMID: 31650025 Free PMC article.
-
Faithful animal modelling of human glioma by using primary initiating cells and its implications for radiosensitization therapy [ARRIVE 1].Sci Rep. 2018 Sep 21;8(1):14191. doi: 10.1038/s41598-018-32578-w. Sci Rep. 2018. PMID: 30242200 Free PMC article.
-
Interferon regulatory factor-1 suppresses DNA damage response and reverses chemotherapy resistance by downregulating the expression of RAD51 in gastric cancer.Am J Cancer Res. 2020 Apr 1;10(4):1255-1270. eCollection 2020. Am J Cancer Res. 2020. PMID: 32368400 Free PMC article.
-
Radiopotentiation Profiling of Multiple Inhibitors of the DNA Damage Response for Early Clinical Development.Mol Cancer Ther. 2021 Sep;20(9):1614-1626. doi: 10.1158/1535-7163.MCT-20-0502. Epub 2021 Jun 22. Mol Cancer Ther. 2021. PMID: 34158341 Free PMC article.
References
-
- Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3:255–68. - PubMed
-
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. - PubMed
-
- Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nature reviews Cancer. 2010;10:319–31. - PubMed
-
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30NS047463/NS/NINDS NIH HHS/United States
- R01NS064593/NS/NINDS NIH HHS/United States
- T32 CA113277/CA/NCI NIH HHS/United States
- P30 NS047463/NS/NINDS NIH HHS/United States
- P30CA016059/CA/NCI NIH HHS/United States
- P30 CA016059/CA/NCI NIH HHS/United States
- T32CA113277/CA/NCI NIH HHS/United States
- R01 NS064593/NS/NINDS NIH HHS/United States
- T32CA085159/CA/NCI NIH HHS/United States
- T32 CA085159/CA/NCI NIH HHS/United States
- F30 CA171893/CA/NCI NIH HHS/United States
- R21 CA156995/CA/NCI NIH HHS/United States
- F30CA171893/CA/NCI NIH HHS/United States
- R21CA156995/CA/NCI NIH HHS/United States
- S10 RR022443/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

